0552
Downloadable Probabilistic Map of the Territorial Distribution of the Left – Middle Cerebral Artery Derived from TOF-MRA1Médecine Nucléaire et Radiobiologie, Université de Sherbrooke, Sherbrooke, QC, Canada, 2Pédiatrie, Université de Sherbrooke, Sherbrooke, QC, Canada, 3Radiologie Diagnostique, Université de Sherbrooke, Sherbrooke, QC, Canada
Synopsis
Standardized artery territorial distributions (ATD) are derived from variable post-mortem ATD yet assume a homogenous distribution. We developed a downloadable probabilistic territorial distribution of the left-MCA derived from Time-of-Flight Magnetic-Resonance-Angiography that can be used with other MRI modalities. We examined the probability of the arterial territory in Broca’s and Wernicke’s area and found it to be almost 3 times higher in Broca area than Wernicke’s area; however, both are traditionally believed to be supplied by the left-MCA. Combining the variability of arterial territories with functionally defined regions of interest can advance our knowledge of the consequences of cerebrovascular incidents.
Introduction
Blood is supplied to the brain through three major cerebral arteries: the middle cerebral arteries (MCA), anterior cerebral arteries and posterior cerebral arteries each with their respective arterial territory1. Standardized arterial territorial distributions (ATD) of the major cerebral arteries are largely based on post-mortem studies and distribution of the arterial territories are presented as homogeneous2. However, the original post-mortem maps from which the ATD are based on vary markedly between studies suggesting that the arterial territories are in fact not uniform2. This is not unexpected as the location and size of collaterals of cerebral arteries are highly variable3 and the probability of lesion after stroke within the same artery is not uniform4. Therefore, a probabilistic ATD highlighting the variability of cerebral arteries could be useful for studying cerebrovascular incidents and their relationship to immediate and long-term neurological outcomes1,5.Recent advancement in Magnetic Resonance Angiography (MRA) using non-contrast enhanced Time of Flight (TOF) imaging allow us to non-invasively image cerebral arteries which can be segmented to generate a 3D image of the cerebral vascular tree3,6. These techniques allow us to isolate and study the regions surrounding the cerebral arteries in conjunction with other MRI modalities. The MCA is of particular interest as its collaterals are more frequently involved in cerebrovascular events1. Therefore, we propose a probabilistic ATD which takes into consideration the high variability of the left-MCA and its collaterals derived from in vivo images of cerebral arteries.
Methods
Nine participants (5 females) were imaged on a 3T Insignia scanner equipped with a 32-channel head-coil (Philips Healthcare, Best, Neatherlands). First, a 3D gradient-echo T1 weighted image was collected (field of view (FOV)=240X240X161 mm; TR/TE=7.9/3.5ms, 1 mm isotropic voxels, flip angle(FA)= 8°), followed by a high resolution, whole-brain multi-band TOF image (FOV= 200X200X120.9mm, TR/TE= 23/3.45ms, FA= 18°, parallel imaging (SENSE) acceleration factor=3, acquisition resolution of 0.65X0.65X1.30, reconstructed resolution of 0.626x0.625x0.65mm). Each participant’s vascular tree was segmented from TOF images using a method developed in our laboratory3,6 and extracted to create a 3D image of the cerebral vascular tree from which arterial diameters were computed (Figure 1A). The arterial tree was then registered to the participant’s T1 image and warped to the MNI 152 asymmetric brain template7,8. To reduce false-positive identification of arteries, only those with a diameter of 0.5mm were examined. Next, the region around the left-MCA and its collaterals were manually inspected and masked using FSLeyes9 mask tool with a 10mm isotropic box (figure 1.C).The masks were then averaged to create a probabilistic ATD. Next, the probabilistic ATD was segmented into cerebral spinal fluid (CSF) white matter (WM) and gray matter (GM) using CSF, GM and WM mask of MNI 152 T1 segmented with FSL (tissue class probability of >50%)10–12. A binary mask of the ATD was created to show the anatomical boundaries of our ATD (Figure 1D) and to examine the vascular density (VD) in the entire map and in the GM, WM, and CSF. Since language processing is often affected by left-MCA stroke, we examined the probability of left-MCA branches within Broca’s and Wernicke’s areas.Results
The probabilistic ATD is shown in figure 2. The ATD was divided into three tissue class: CSF (Figure 2A), WM (Figure 2B), GM (Figure 2C) and all tissues (Figure 2D). The probability of an artery ranged from 11% to 100% and was, on average, highest in the CSF (47.52 ± 10.2%; where the largest part of the left-MCA is located) followed by GM (44.75 ± 9.10%) and WM (31.45 ± 7.18%). The anatomical regions with the highest probability were the insula, parts of inferior frontal gyrus and the superior temporal gyrus. The VD within the entire probabilistic map was 31.82 ± 1.4% and was highest in the CSF (47.36 ± 1.16%), then GM (30.84 ± 1.42%) and lowest in the WM (19.01 ± 1.73%). Broca’s area had an average probability of 64.39 ± 7.46% while Wernicke’s area had an average probability of 27.35 ± 5.25% (figure 3).Discussion
Here we propose a probabilistic ATD of the territorial distribution of the left-MCA derived from non-invasive TOF images of the cerebral vascular tree. The boundaries of our probabilistic ATD lies within previously derived ATDs2,13. Additionally, our probabilistic ATD is similar to Thye & Mirman's (2018) left-MCA stroke lesion overlap map4. These similarities confirm that the artery and surrounding tissue isolated during the masking process did indeed belong to the left-MCA. Broca’s and Wernicke’s aphasia are both associated to left-MCA stroke1. Our results confirmed that Brocas’s area is well perfused by the left-MCA as it overlapped with regions of high probability in our ATD. Conversely, the low probability found for Wernicke’s area suggests that the left-MCA may not be the only source of blood to this region or that it is supplied by arteries below our imaging resolution. This result highlights that our probabilistic ATD can be used with other MRI modalities when studying different neurological disorders and cerebrovascular incidents to grain additional insight on the source and variability of cerebral arteries of functionally defined regions.Acknowledgements
No acknowledgement found.References
1. Blumenfeld, H. Neuroanatomy Through Clinical Cases. (2010).
2. Zwan, A. Van Der & Hillen, B. Comments, Opinions, and Reviews Review of the Variability of the Territories of the Major Cerebral Arteries. 1078–1085 (1991).
3. Bernier, M., Cunnane, S. C. & Whittingstall, K. The morphology of the human cerebrovascular system. Hum. Brain Mapp. 1–14 (2018). doi:10.1002/hbm.24337
4. Thye, M. & Mirman, D. Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. NeuroImage Clin. 20, 1129–1138 (2018).
5. The Lancet Neurology. Vascular disease and neurodegeneration: advancing together. Lancet Neurol. 16, 333 (2017).
6. Bizeau, A. et al. Stimulus-evoked changes in cerebral vessel diameter: A study in healthy humans. J. Cereb. Blood Flow Metab. 38, 528–539 (2018).
7. Fonov, V. et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54, 313–327 (2011).
8. Fonov, V., Evans, A., McKinstry, R., Almli, C. & Collins, D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 47, S102 (2009).
9. McCarthy, P. FSLeyes. (2019). doi:10.5281/ZENODO.3530921
10. Woolrich, M. W. et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage 45, S173–S186 (2009).
11. Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
12. Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
13. Tatu, L., Moulin, T., Vuillier, F. & Bogousslavsky, J. Arterial territories of the human brain. Front. Neurol. Neurosci. 50, 1699–1708 (1998).
Figures
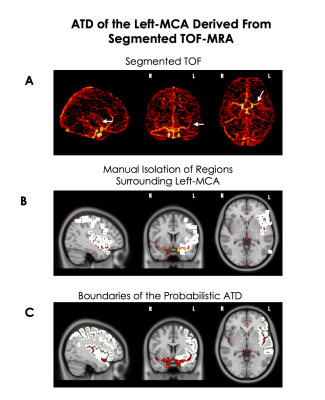
Figure 1
A) Maximum intensity projection of a segmented whole-brain TOF from one participant
B) Masking of tissue surrounding the left-MCA and collaterals from one participant. A 10x10x10 voxel box was placed whenever an artery was present in order to isolate tissue surrounding the artery.
C) Binary mask of the average mask of tissue surrounding MCA and Collaterals across 9 participants. The mask shows the gray matter boundaries of the perfusion territory overlaid in relation to the left-MCA.
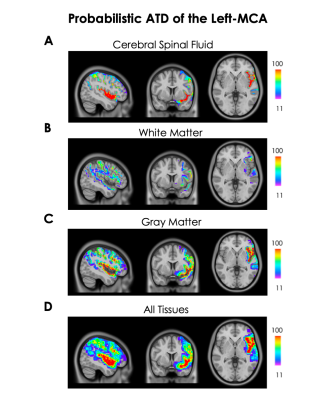
Figure 2.
A) Probability of left-MCA and collaterals in the CSF.
B) Probability of left-MCA and collaterals in the WM.
C) Probability of left-MCA and collaterals in the GM.
D) Probabilistic ATD of the left-MCA across all tissue types. Note the large variability in the probability of the territory of the left-MCA. The regions where the left-MCA's diameters are largest have the highest probability.
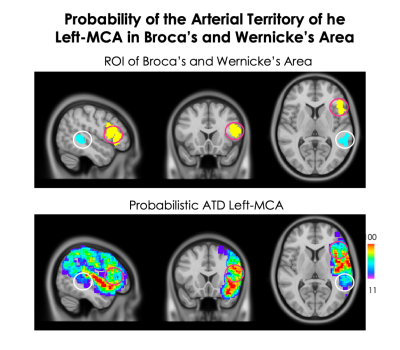
Figure 3.
Comparison of the probability of MCA arterial territory in Broca’s area (yellow ROI, pink circle) and Wernicke’s area (blue ROI, white circle). Broca’s area had an average probability of 64.39 ± 7.46% while Wernicke’s area had an average probability of 27.35 ± 5.25% yet both are associated with left-MCA stroke1. This would suggest that Broca’s area likely receives most of its blood supply from the left MCA whereas Wernicke’s area may receive blood from small MCA collaterals below our imaging resolution or be supplied by another cerebral artery.