0548
Post-mortem Diffusion MRI Analysis of Neuronal Pathways in the Human Hippocampus
Choong Heon Lee1, Jing Li2, Yulin Ge1, Timothy M Shepherd1, Youssef Zaim Wadghiri1, Jiangyang Zhang1, and David W Nauen3
1Radiology, New York University School of Medicine, New York, NY, United States, 2Peking Union Medical College Hospital, Beijing, China, 3Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
1Radiology, New York University School of Medicine, New York, NY, United States, 2Peking Union Medical College Hospital, Beijing, China, 3Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
High-resolution diffusion MRI data of post-mortem adult human hippocampus specimens were acquired and compared to histology to identify major axonal pathways in the hippocampus. The complex microstructural organization in the hippocampus made it difficult to resolve axonal pathways based on conventional diffusion tensor data. In comparison, neurite density map using the NODDI toolbox revealed the locations of the perforant path, mossy fibers, and Schaffer collaterals confirmed by histology. We were able to reconstruct the fimbria/alveus and perforant pathways using tractography, and the results resembled in vivo results from the HCP dataset. Other pathways in the hippocampus remained difficult to delineate.
Introduction
The hippocampus plays an important role in memory and learning1. It consists of several subregions, which have unique cyto-architectural features. Axonal pathways including the perforant path, mossy fibers, and Schaffer collaterals convey information among these subfields and other brain regions, mainly the entorhinal cortex. It has long been postulated that the morphology and microstructural organization of hippocampal areas and the structural integrity of the hippocampal pathways may serve as markers of present or future memory ability. While software tools have been developed for segmentation of hippocampal subfields2,3, and diffusion MRI (dMRI) tractography has been used to trace some hippocampal pathways4 and correlate dMRI signals with memory function5, visualization of microstructural organization, especially that of axonal pathways, within the hippocampal subfields remains limited. Several studies have used post-mortem structural and diffusion MRI to examine the human hippocampus at high spatial resolution 6-10. In this study, we combined high-resolution diffusion MRI and histology to examine neural pathways in the hippocampus.Methods
The study was approved by the Institutional Review Board. De-identified post-mortem specimens of formalin-fixed adult human hippocampus (age 16-44 years, n=3) were obtained after pathological autopsy examination following family consent. Experiments were performed on a horizontal 7-T MRI system (max gradient strength=600mT/m) using a 30 mm diameter volume transmit/receive coil at room temperature with the following parameters: 1) 2D multi-shell dMRI: 4-shot EPI, TE/TR = 38.2/6000 ms, δ/Δ = 6/24 ms, BW=200KHz, 60 diffusion directions with 6 b shells from 2,000 to 12,000 s/mm2; 2) 3D single-shell dMRI: 3D diffusion-weighted GRASE, TE/TR = 40/500 ms, d/D = 6/20 ms, 0.25 mm isotropic resolution, BW=200KHz, 60 diffusion directions, b = 5000 s/mm2; 3) 3D T2-weighted MRI: RARE, TE/TR = 50/2000ms, ETL=8, 0.25 mm isotropic resolution. dMRI data were analyzed using MRtrix and NODDI11 toolboxes. Tissue sections were stained with hematoxylin and eosin (H&E, cellular structure), neurofilament (EMD Millipore, clone SMI-31, axons), and Hirano silver (cell bodies and processes).Results
Normalized diffusion-weighted signal attenuation curves (Fig.1) show that gray matter had faster signal decays than white matter, but the contrast mostly stabilized for diffusion weighting (b) greater than 5,000 s/mm2. Results from fitting the 2D multi-shell dMRI data to diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI) models were compared with histology at similar locations (Fig. 2). Based on the DTI, histology, and previous reports, we segmented hippocampal subfields in the 3D dMRI volume (Fig. 2). The dentate gyrus had higher mean diffusivity (MD) values than surrounding regions. The CA1 region (Fig. 3, white arrows) showed moderate fractional anisotropy (FA) values and consistent radial orientation, likely due to coherently arranged apical dendrites in this region, as shown by silver staining.The neurite density index (NDI) and orientation dispersion index (ODI) maps revealed additional information on hippocampal pathways. The NDI map showed several neurite areas in the CA1-CA3 subregions (Fig. 3, red, blue, and green arrows), in good agreement with SM31 stained sections showing axons in the perforant path (blue), Schaffer collaterals (red), and mossy fibers (green). The same regions had low diffusion anisotropy and are difficult to visualize using DTI, probably due to crossing of these axons with apical dendrites of the pyramidal neurons. This is supported by high orientation dispersion in these regions shown by the ODI map.
Using fiber orientation distribution (FOD) maps (Fig. 4A) estimated from the 3D dMRI volume and subfield segmentation (Fig. 2), reconstruction of the fimbria/alveus (Fig. 4B), perforant pathway (Fig. 4C), and mossy fiber pathway (Fig. 4D) were compared to in vivo results from the Human Connectome Project data (not shown here). Our results suggest that fimbria/alveus and perforant pathways can be reconstructed from dMRI data but the mossy fiber and other intrahippocampal pathways remain challenging to reconstruct due to the large population of orthogonally-oriented apical dendrites.
Discussions
Our results demonstrate that NODDI can reveal additional microstructural information related to neural pathways in the human hippocampus. Previous reports have described similar findings in hippocampal subfields10. The histological data reported in this study further confirms the NODDI results. It is necessary to note that NODDI results depend on initial setting of intra and extracellular diffusivities.Our results also suggest that several intra-hippocampal pathways are difficult to reconstruct using conventional dMRI tractography tools due to the presence of apical dendrites. The limitations in identifying these structures can also be attributed to tissue degradation after death. However, little degradation was noted in the current case based on the corresponding histological sections. This limitation raises the need to further refine the tractography tools utilizing additional information provided by NODDI and other tissue microstructural models.
Conclusion
Despite improvement on the spatial resolution at 0.25 mm, some of the hippocampal pathways remained difficult to reconstruct. However, NODDI proved very useful in providing additional information to delineate neural pathways in the hippocampus.Acknowledgements
No acknowledgement found.References
- Bird CM, et al. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008 Mar;9(3):182-94.
- Iglesias, JE, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage, 2015, Jul; 115:117-137
- Adler DH, et al. Characterizing the human hippocampus in aging and Alzheimer's disease using a computational atlas derived from ex vivo MRI and histology. Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):4252-4257.
- Zeineh MM, et al. Ultra-high resolution diffusion tensor imaging of the microscopic pathways of the medial temporal lobe. Neuroimage, 2012 Sep;62(3): 2065-82
- Yassa MA, et al. Ultra-high resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. PNAS 2010 Jul;107(28):12687-12691.
- Adler DH, et al. Histology-derived volumetric annotation of the human hippocampal subfields in postmortem MRI. Neuroimage 2014 Jan 84:505-23.
- Shepherd TM, et al. Diffusion tensor microscopy indicates the cytoarchitectural basis for diffusion anisotropy in the human hippocampus. AJNR Am J Neuroradiol. 2007 May; 28(5):958-64.
- Augustinack JC, et al. Direct visualization of the perforant pathway in the human brain with ex vivo diffusion tensor imaging. Font Hum Neruosci 2010 May 28;4:42.
- Modo M, et al. Detection of aberrant hippocampal mossy fiber connections: Ex vivo mesoscale diffusion MRI and microtractography with histological validation in a patient with uncontrolled temporal lobe epilepsy. Hum Brain Mapp. 2016 Feb; 37(2): 780-795.
- Beaujoin J, et al. Post-mortem inference of the human hippocampal connectivity and microstructure using ultra-high field diffusion MRI at 11.7 T. Brain Struct Funct. 2018 Jun; 223(5):2157-2179.
- Zhang H, et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012 Jul 61(4):1000-16.
Figures
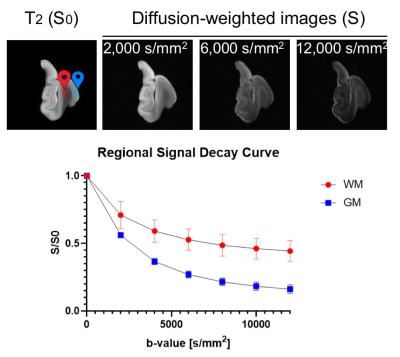
Fig. 1: Diffusion-weighted signal attenuation of post-mortem human
hippocampus specimens (n=3).
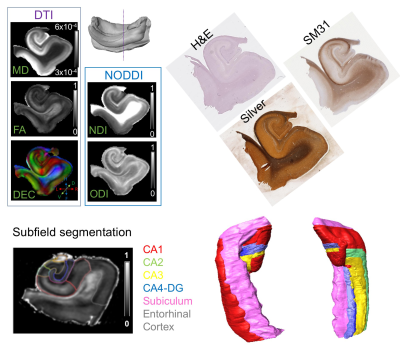
Fig. 2: Comparisons of DTI and NODDI results from
post-mortem diffusion MRI data with histology (H&E, Silver, and SM31).
Manual segmentation of hippocampal subfields are shown with 3D surface
renderings.
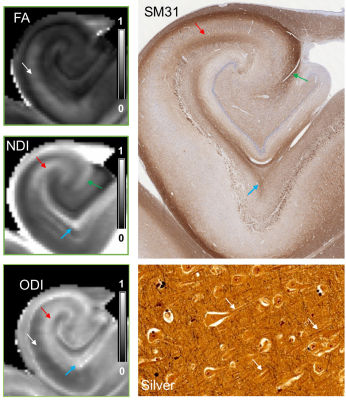
Fig. 3: NODDI results revealed intra-hippocampal
pathways in the hippocampus. The perforant pathway (blue), Schaffer collaterals
(red), and mossy fiber pathway (green) can be delineated in the NDI map and
SM31 histology. The ODI map shows large orientation dispersion in these regions,
probably due to the presence of apical dendrites shown by silver staining,
which also caused low FA values.
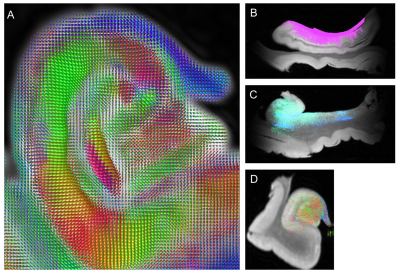
Fig. 4: Fiber orientation distribution map
estimated from 3D dMRI data (A) and reconstruction of the fimbria/alveus (B),
perforant pathway (C), and mossy fiber pathway (D).