0544
Feasibility for MR Elastography to Meet Unmet Need in Intracerebral Hemorrhage Surgical Planning1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 3Neurological Surgery, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
It is hypothesized that the proper surgical approach for intracerebral hemorrhage (ICH) victims should depend on clot rigidity. Neurosurgical experience indicates that brain clot rigidity varies across patients and varies spatially and temporally within each patient. We hypothesize that the wide range of clot rigidity in ICH will allow MR elastography (MRE) techniques to depict the heterogeneity over a wide dynamic range of rigidity. Longitudinal MRE, ultrasound elastography, and mechanical compression testing were performed on large ex-vivo swine blood clots. MR elastography shows promise for characterizing the rigidity of intracerebral hemorrhage as indicated by these ex-vivo tests.
Introduction
Over 5 million people worldwide will suffer an intracerebral hemorrhage (ICH) this year (1). Common neurosurgical experience indicates that brain clot rigidity varies across patients and varies spatially and temporally within each patient. During the crucial days after onset, liquid blood first coagulates into a gel-like structure and later into quite rigid components before eventually breaking down weeks later. It has long been hypothesized that the proper surgical approach should depend on rigidity, with minimally invasive devices administering suction to remove gel-like sections and slowly administering clot-busting drugs into more rigid ones(1, 2).While multiparametric MRI can age hemorrhage onset, neither conventional MRI nor other non-invasive modalities have proven effective in providing an indication of clot rigidity. While MR elastography (MRE) has identified subtle differences across populations of degenerative brain disease(3), we hypothesize that the wide range clot rigidity in ICH will allow MRE to depict the heterogeneity over a wide dynamic range of rigidity. To gain insight prior to a clinical trial, we performed longitudinal MRE, ultrasound elastography and mechanical compression testing on large ex-vivo swine blood clots.
Methods
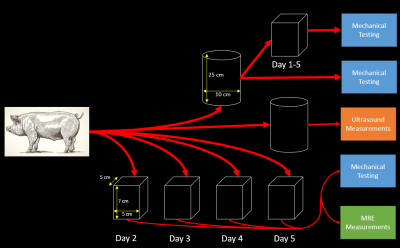
All experiments were performed on naturally clotted swine blood obtained from UW Animal Sciences. A flowchart, shown in Fig. 1, demonstrates the methodology that generated MRE, mechanical, and ultrasound measurements once per day over five days. Unconstrained free plasma was removed from boxes before MRE measurements were obtained.Shear waves were induced with a 60 Hz paddle pneumatic driver set at 70% driver amplitude (Resoundant, Rochester, MN). An MR Touch\(^{TM}\) protocol was altered for shorter data acquisition intervals due to the short T2* of clotted blood on a 3.0T GE Premier scanner (GE Healthcare, Waukesha, WI) at 4 temporal phases. The protocol produced 3.0mm slices at 2.5 mm spacing with 120 Hz motion encoding gradients.
Clots were tested in confined compression on a tabletop test machine (TA Instruments, 3230AT Series III). Clot samples (approx. 1 cm thick, 14 mm diameter) were extracted from the overall clot daily before MRE. Instantaneous modulus was calculated from the initial ramp to 10% strain at 0.25 mm/s. Multiple samples were tested at each time point.
Ultrasound shear modulus measures were performed using the Virtual Touch Mode Imaging on an S2000 Siemens Ultrasound (Mountain View, CA) on evolving blood stored in the 2 L cylinder shown in Figure 1. Fifteen to twenty measurements were made each day at a depth of 2 cm, shifting the transducer to a new location of the clot surface approximately every 5-8 measurements. Shear modulus (\(\mu\)) was calculated using \(\mu = \rho*V_{s}^{2}\) where \(\rho\) is blood density (\(1060 \frac{k_{g}}{m^{3}}\)) and \(V_{s}\) is shear wave speed.
Results
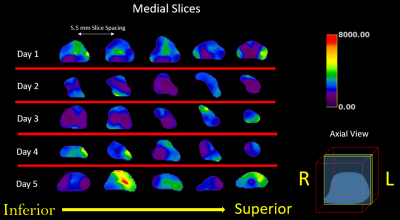
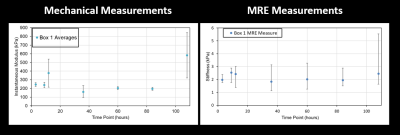
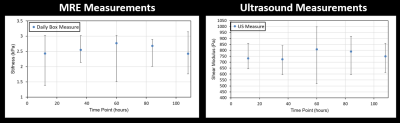
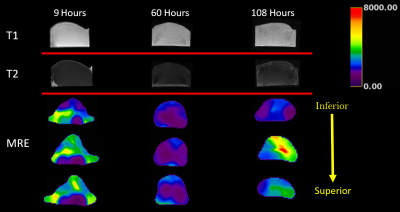
A series of axial MRE slices from the same box are illustrated in Figure 2. Note the spatial heterogeneity of clot stiffness within each slice and the evolution of each slice over the five day trial. During the first 12 hours of natural clotting, average stiffness throughout the clot varied from 1.25 kPa to 3.52 kPa. Over the next 48 hours, overall stiffness of the clot drops, but slowly increases up to areas as stiff as 7.4 kPa by Day 5.Figure 3 shows a similar trend in clot evolution in compression-based stiffness and MRE shear wave measures over a five day period. Longitudinal ultrasound-based measurements are compared to MRE over the five day trial in Figure 4. For both Figs. 3 and 4, a single measure of MRE-derived stiffness across the entire box was measured by calculating the average clot stiffness value on each slice and then averaging for a single bulk measurement. The comparison in Fig. 3 and 4 show similar trends in evolution of stiffness. Given the significant spatial heterogeneity of clots, greater care must be taken to co-register the spatial location of the core compression sample within each MRE exam before generating a measure of correlation.
While T1 and T2 imaging is spatially isointense during the five day trial, Figure 5 demonstrates widely varying stiffness spatially and temporally.
Discussion
MRE demonstrated its ability to depict wide spatial and temporal heterogeneity in the longitudinal evolution of blood clots. Rapid EPI acquisitions are necessary due to T2* signal decay. The large concentration of iron in blood clots creates similar but much larger difficulties than experienced in MRE of the liver with iron overload.As MRE and USE measure shear modulus, these measures are related but not the same as the elastic modulus measured by compression testing. Future work will map MRE values to surgically relevant predictions of clot rigidity. The spatial distortion caused by B0 inhomogeneity at air/tissue interfaces also demands changes in our experimental setting to surround the blood clots with some signal-creating material. As freezing and microtoming to create sections will alter rigidity, an image-guided procedure to core sub-regions for mechanical testing that can be registered to MRE might be necessary.
Conclusion
MR elastography shows promise for characterizing the rigidity of intracerebral hemorrhage as indicated by these ex-vivo tests. The work identified confounding factors to be addressed in another study and generated enthusiasm for an add-on human MRE study after onset of ICH.Acknowledgements
We gratefully acknowledge the technical support of Resoundant and Dr. Kevin Glaser of the Mayo Clinic with MRE measurements. Rashid-Al Mukaddim is thanked for his help with the ultrasound elastography measurements as is Dr. Alan McMillan with MRE and Jennifer Meudt for assistance in obtaining swine blood. We also acknowledge GE Healthcare Research support.References
1. Hanley DF, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15(12):1228-37. doi: 10.1016/S1474-4422(16)30234-4. PubMed PMID: 27751554.
2. Hanley DF, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019. Epub 2019/02/12. doi: 10.1016/S0140-6736(19)30195-3. PubMed PMID: 30739747.
3. Yin Z, Romano AJ, Manduca A, Ehman RL, Huston J, 3rd. Stiffness and Beyond: What MR Elastography Can Tell Us About Brain Structure and Function Under Physiologic and Pathologic Conditions. Top Magn Reson Imaging. 2018;27(5):305-18. Epub 2018/10/06. doi: 10.1097/RMR.0000000000000178. PubMed PMID: 30289827; PMCID: PMC6176744.
Figures