0542
Venous Mapping of Vascular Malformations using Cranial 4D Flow MRI with Improved ‘Virtual Injections’1Radiology, University of Wisconsin - Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 3Radiology, Stanford University, Palo Alto, CA, United States
Synopsis
Endovascular intervention via a venous approach, or trans-venous embolization (TVE), has been increasing employed in the management of intracranial vascular lesions such as arteriovenous malformations (AVMs) and dural arteriovenous fistulas (DAVFs). Current pre-procedural planning is limited by overlapping, complex vascular anatomy and a lack of quantitative hemodynamic feature characterization. Using novel 4D flow MRI methods, high-resolution retrograde venous flow mapping with anatomical detail and dynamic flow fields can provide valuable information prior to TVE. We will present our institutional experience using this method in representative intracranial vascular lesions.
Introduction
The management of intracranial vascular lesions such as arteriovenous malformations (AVMs) and dural arteriovenous fistulas (DAVFs) can be complex with significant morbidity and mortality. Endovascular intervention via a venous approach, or trans-venous embolization (TVE), is a common method to treat DAVFs. TVE for AVM treatment has recently gained interest; however, accurate characterization of venous drainage is essential to this interventional approach yet not readily available. 4DFlow MRI has made significant advances over the last decade and can provide anatomical detail and dynamic flow fields with high resolution. One compelling yet underutilized use of these unique comprehensive datasets is the concept of ‘virtual injections’ to track the path of the blood flow from the flow data alone without the need for an actual injection1 with widespread potential clinical applications. In this work, we apply a novel method2 that combines probabilistic3 displacement corrections4, and fluid constraints to track blood near AVMs and DAVFs using only 4D flow acquisitions. Utilizing our method, these ‘virtual injections’ can be performed both antegrade and retrograde through the vasculature, which can greatly aid in lesion characterization prior to entering the angiography suite. Here we will present our institutional experience using this novel 4D Flow MRI based methodology to perform comprehensive venous mapping of intracranial vascular malformations, providing valuable insight into the pre-procedural vascular anatomy and the potential impact of selective embolization.Methods
A total of 11 AVM and 2 DAVF cases were imaged with 4D flow MRIon clinical 3T scanners (Discovery 750, GE Healthcare). 4D flow data was acquired with a radially-undersampled PCVIPR5 acquisition with complete volumetric brain coverage: scan time=~6min., VENC=80cm/s, isotropic spatial resolution: 0.78 mm. Streamline steps were calculated using a 4th order Runge-Kutta (RK4) method from time-averaged velocity maps of the brain. Streamline starting positions (seeds) were placed within a masked plane in the neck for whole vascular images or from a manually positioned sphere for isolating single vessels for more detailed AVM and DAVF vessel analysis. Every new point of a streamline was calculated with two RK4 steps, where the first step (t = 2.4-2.7 ms) was used to approximately compensate for velocity displacement artifacts, and the second (t=3 ms) was then the actual streamline step to calculate the new position. Displacement corrections were applied for each step. Stochastic noise was accounted for by perturbing each step with a vector randomly sampled from a Gaussian distribution (σ=1cm/s). Additional constraints were imposed to select lines that minimize changes in kinetic energy, as well as preferring lines that stay within the vessel boundaries as determined automatically segmented vessel walls from the PC MR angiogram. For validation, dynamic arterial spin labeling (ASL) images were acquired using a pseudo-continuous labeling (PCASL) sequence6 : scan time=~7min.Results
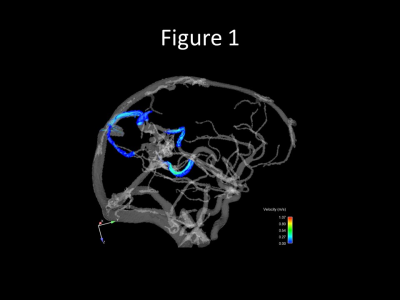
Data acquisition, reconstruction, and processing was successfully completed in all 13 cases. Images of a right temporoparietal AVM are demonstrated in detail in the following example figures:Figure 1 shows a 4D flow generated angiogram and velocity maps.
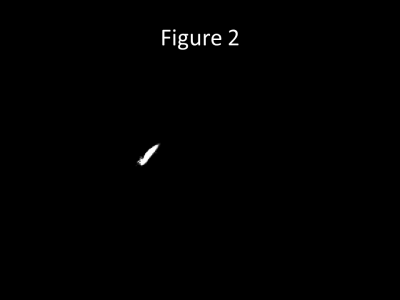
Figure 2 shows ‘virtual injection’ seeds placed in the posterior aspect of the superior sagittal sinus with retrograde flow tracking through the nidus of the right temporoparietal AVM into the feeding arteries.
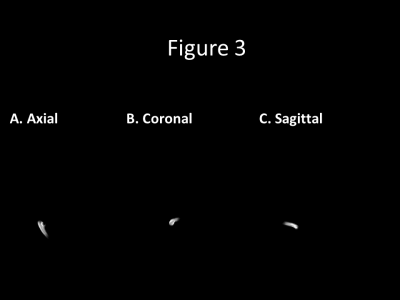
Figure 3 shows (A) axial, (B) coronal, and (C) sagittal animated gifs with seeds placed in the dominate right transverse sinus. There is retrograde flow tracking though the nidus into the feeding arteries. The three views assist in delineating two draining veins; however, the slower draining vein is less clearly visualized.
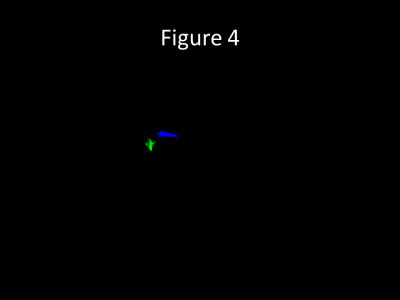
Figure 4 shows an animated gif demonstrating seeds placed within each of the draining veins individually with dual-color representation. There is faster flow through the blue draining vein with quicker retrograde filling of the feeding artery. Slower flow is seen in the green draining vein, which is better accentuated on the dual seeding compared to proximal vein seeding alone (Figures 2 and 3).
Figure 5 shows multiple still images from the figure 4 gif. (A) demonstrates flow tracking with early retrograde flow into the two draining veins. (B) and (C) demonstrate faster retrograde filling of the nidus from the blue vein (white arrow) with early filling of the feeding arteries (white arrowhead), indicating faster flow thought the portion of the nidus drained by the blue vein. (D) demonstrates flow in the portion of the nidus draining into the slower flow green vein now reaching the feeding artery (yellow arrow).
Discussion and Conclusion
This work demonstrates a novel method combining probabilistic streamlines, displacement corrections, and fluid constraints to track blood movement throughout the whole brain using only 4D flow acquisitions and the concept of ‘virtual injections’. Unlike DSA and ASL, seed locations can be (1) chosen retrospectively, (2) at multiple locations, and (3) placed in downstream vessel segments with retrograde tracking. This analysis is complementary to the quantitative flow analysis provided by 4D flow acquisitions and is calculated solely with the MR data. These probabilistic streamlines generated using corrected 4D flow MR data allow for complex venous mapping in vascular malformations which could have high impact by assisting in vascular lesion characterization prior to and after treatment. Future studies are needed to assess the actual impact on improved pre-procedure planning and patient outcomes.Acknowledgements
No acknowledgement found.References
[1] M. Edjlali et al., “MR selective flow-tracking cartography: A postprocessing procedure applied to four-dimensional flow MR imaging for complete characterization of cranial dural arteriovenous fistulas,” Radiology, vol. 270, no. 1, pp. 261–268, 2014.
[2] M. Loecher, K. Johnson, P. Turski, and O. Wieben, “Robust Whole-Brain Blood Tracking from 4D Flow using Displacement Corrected Probabilistic Streamlines,” in Proc Intl Soc Mag Reson Med 22, 2014, p. 0513.
[4] O. Friman, A. Hennemuth, A. Harloff, J. Bock, M. Markl, and H. O. Peitgen, “Probabilistic 4D blood flow tracking and uncertainty estimation,” Med Image Anal, vol. 15, no. 5, pp. 720–728, 2011.
[5] D. A. Steinman, C. R. Ethier, and B. K. Rutt, “Combined analysis of spatial and velocity displacement artifacts in phase contrast measurements of complex flows,” J Magn Reson Imaging, vol. 7, no. 2, pp. 339–346, 1997.
[6] T. Gu et al., “PC VIPR: A high-speed 3D phase-contrast method for flow quantification and high-resolution angiography,” AJNR Am J Neuroradiol, vol. 26, no. 4, pp. 743–749, 2005.
[7] H. Wu et al., “Noncontrast dynamic 3D intracranial MR angiography using pseudo-continuous arterial spin labeling (PCASL) and accelerated 3D radial acquisition,” J Magn Reson Imaging, vol. 39, no. 5, pp. 1320–1326, 2014.
Figures