0533
The human phantom: Comprehensive ultrahigh resolution whole brain in vivo single subject dataset1Medicine & Digitalization, Otto-von-Guericke University, Magdeburg, Germany, 2Biomedical Magnetic Resonance, Otto-von-Guericke University, Magdeburg, Germany, 3German Center for Neurodegenerative Diseases, Magdeburg, Germany, 4Center for Behavioral Brain Sciences, Magdeburg, Germany, 5Leibniz Institute for Neurobiolgoy, Magdeburg, Germany
Synopsis
Here, we present an extension to our previously published T1-weighted dataset with an ultrahigh isotropic resolution of 250 µm, consisting of multiple additional contrasts. Included are up to 150 µm ToF, an updated 250 µm MPRAGE, 330 µm QSM, up to 450 µm T2-weighted SPACE, 750 µm MPM, 800 µm DTI, one hour continuous rs-fMRI as well as more than 130 MPRAGE volumes collected over 10 years (with varying spatial resolution between 450 µm and 1 mm). All data were acquired on the same 7 T scanner and of the same subject. Basic pre-processing of all data were conducted.
Introduction
Previously we published a human whole brain in vivo MRI dataset with an ultrahigh isotropic resolution of 250 µm [1], freely available elsewhere [2,3]. Here, we present an extension to this dataset containing multiple additional contrasts with ultrahigh resolution and (mostly) full brain coverage. Included are up to 150 µm ToF [4], 250 µm MPRAGE [1], 330 µm QSM [5], up to 450 µm T2-weighted SPACE, 750 µm MPM [6], 800 µm DTI, one hour continuous 1.8 mm rs-fMRI and more than 130 MPRAGE volumes collected over 10 years (with varying isotropic spatial resolution between 450 µm and 1 mm). All data were acquired on the same 7 T scanner and of the same subject.Methods & Materials
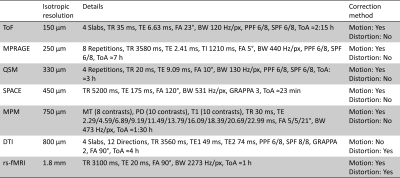
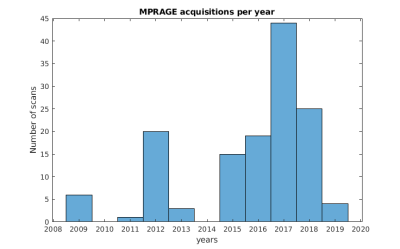
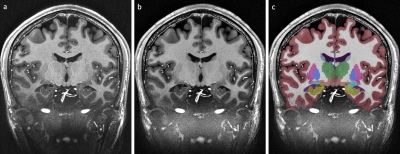
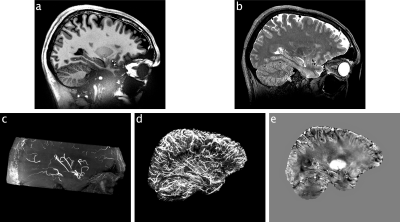
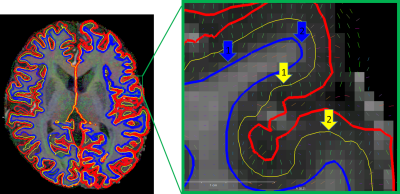
Data were acquired of one healthy Caucasian male subject (born 1982) with no known neurodegenerative or psychiatric disease. The first data were collected in 2009 and the latest in 2019 (Fig. 1). The subject gave written informed consent prior to each study and, retrospectively, to share all data publicly. The local ethics committee has approved each study.All data were acquired at the same 7 T MR scanner (Siemens 7T Classic, Erlangen, Germany). From 2009 to 2011 data were acquired with a 1 Tx/24 Rx channel head coil (Invivo, Gainsville, FL, USA) and from 2012 onwards with a 1 Tx/32 Rx channel head coil (Nova Medical Inc, MA, USA). Many data were acquired with prospective motion correction (MT384i, Metria Innovation Inc., WI, USA) [7]. The DTI and rs-fMRI data were distortion corrected by using a point-spread function approach [8]. With the exception of the time of flight acquisition, all protocols cover the entire brain. All non-MPRAGE data were rigidly registered to the previously published 250 µm MPRAGE using ANTs [9]. All MPRAGE data were quality checked with MRIQC [10], bias field corrected with SPM12 [11] and processed with FreeSurfer [12]. In figure 1, the most important imaging parameters of the ultrahigh resolution acquisitions are given per study and figures 2 shows a histogram of the MPRAGE acquisitions per year. The figures 3 to 5 display images of the updated MPRAGE volume, the different contrasts registered to the MPRAGE and the DTI, respectively.
Discussion
This unique dataset will be made publicly available shortly, scheduled for early 2020.The more than 130 MPRAGE volumes allow for longitudinal processing of the data. However, some of the MPRAGE acquisitions may not be suited for quantification, e.g. volumetry or cortical thickness measures, as some volumes were acquired for testing protocols. These may be removed in the release of the dataset potentially.
Acknowledgements
This work received funding from the federal state of Saxony-Anhalt under grant number ‘I 88’, from NIH under grant number ‘1R01-DA021146’, from DFG under grant number 'SP632/3', by the Initial Training Network, HiMR, funded by the FP7 Marie Curie Actions of the European Commission, grant number 'FP7-PEOPLE-2012-ITN-316716', by the Federal Ministry of Education and Research of Germany within the Forschungscampus STIMULATE, grant number '13GW0095A' and from Wellcome Trust under grant number '203147/Z/16/Z'.
References
1. Lüsebrink F, Sciarra A, Mattern H, Yakupov R, Speck O. T1-weighted in vivo human whole brain MRI dataset with an ultrahigh isotropic resolution of 250 μm. Sci Data. 2017;4:170032 doi: 10.1038/sdata.2017.32.
2. Lüsebrink F, Sciarra A, Mattern H, Yakupov R, Speck O. Raw data from: T1-weighted in vivo human whole brain MRI dataset with an ultrahigh isotropic resolution of 250 μm doi: 10.24352/UB.OVGU-2017-001.
3. Lüsebrink F, Sciarra A, Mattern H, Yakupov R, Speck O. Data from: T1-weighted in vivo human whole brain MRI dataset with an ultrahigh isotropic resolution of 250 μm doi: 10.5061/DRYAD.38S74.
4. Mattern H, Sciarra A, Godenschweger F, Stucht D, Lüsebrink F, Rose G, Speck O. Prospective motion correction enables highest resolution time-of-flight angiography at 7T. Magn Reson Med. 2018;80(1):248–258 doi: 10.1002/mrm.27033.
5. Mattern H, Sciarra A, Lüsebrink F, Acosta-Cabronero J, Speck O. Prospective motion correction improves high-resolution quantitative susceptibility mapping at 7T. Magn Reson Med. 2019;81(3):1605–1619 doi: 10.1002/mrm.27509.
6. Weiskopf N, Suckling J, Williams G, Correia MM, Inkster B, Tait R, Ooi C, Bullmore ET, Lutti A. Quantitative multi-parameter mapping of R1, PD(*), MT, and R2(*) at 3T: a multi-center validation. Front Neurosci. 2013;7:95 doi: 10.3389/fnins.2013.00095.
7. Maclaren J, Armstrong, Brian S R, Barrows RT et al. Measurement and correction of microscopic head motion during magnetic resonance imaging of the brain. PLoS ONE. 2012;7(11):e48088 doi: 10.1371/journal.pone.0048088.
8. In M-H, Speck O. Highly accelerated PSF-mapping for EPI distortion correction with improved fidelity. MAGMA. 2012;25(3):183–192 doi: 10.1007/s10334-011-0275-6.
9. Avants BB, Tustison NJ, Johnson HJ. Advanced Normalization Tools (ANTs) [Internet]; 2015. Available from: http://stnava.github.io/ANTs/.
10. Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS ONE. 2017;12(9):e0184661 doi: 10.1371/journal.pone.0184661.
11. Ashburner J. SPM: a history. Neuroimage. 2012;62(2):791–800 doi: 10.1016/j.neuroimage.2011.10.025.
12. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781 doi: 10.1016/j.neuroimage.2012.01.021.
13. Lüsebrink F, Mattern H, Oeltze-Jafra S, Speck O. Beyond high resolution: Denoising during image reconstruction to improve image quality. In: ESMRMB 2019, Rotterdam.
Figures