0529
Variable Flip Angle 3D Echo Planar Time-Resolved Imaging (vFA 3D-EPTI) for Fast Multi-Compartment Quantitative Mapping1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States, 3Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 4Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, Netherlands
Synopsis
Multi-compartment models have been developed to detect the microstructure properties of brain tissue using multi-modal MRI, but are limited by the long scan time of multi-contrast multi-parametric acquisition. In this work, a novel variable flip angle EPTI (vFA 3D-EPTI) technique is developed to quickly acquire rich multi-contrast information for multi-compartment analysis. The optimized ‘temporal variant CAIPI’ sampling was used, and an augmented subspace reconstruction with multi-compartment modelling is also developed to accurately reconstruct complex signal evolution. Through this approach, myelin water fraction, proton density, multi-compartment T1, T2* maps can be acquired simultaneously in 12 minutes at 1-mm isotropic resolution.
Introduction
Recently, many multi-compartment models have been developed to detect the sub-voxel microstructure properties of brain tissue using multi-modal MRI1-3. However, to perform multi-compartment analysis, multi-contrast multi-parametric dataset with high-SNR is required which leads to extremely long scan time and limited spatial resolution.Echo planar time-resolved imaging (EPTI)4 is an efficient multi-shot EPI acquisition technique for multi-contrast and quantitative imaging, which not only achieves distortion- and blurring-free imaging, but also resolves hundreds of T2/T2*-weighted images across the EPI readout. The recent extension of EPTI to 3D-EPTI5,6 further improves its acquisition efficiency for multi-parametric quantitative mapping at high spatial resolution.
In this work, we develop a novel variable flip angle 3D-EPTI (vFA 3D-EPTI) technique to acquire multiple flip angles and multi-echo dataset to provide rich information for multi-compartment model fitting. The optimized ‘temporal variant CAIPI’ sampling strategy5 was used to achieve high undersampling in k-t space, with additional complementary k-t samplings across FAs. Moreover, a joint subspace reconstruction is developed to achieve accurate complex signal evolution estimation by incorporating a multi-compartment model into basis generation. Here, an extended complex 3-compartment model for myelin water fraction imaging with T1 correction7 is utilized. Enabled by the high acceleration achieved with the proposed method, myelin water fraction (MWF), proton density (PD), multi-compartment T1, T2* maps can be acquired simultaneously in 12 minutes at 1-mm isotropic resolution.
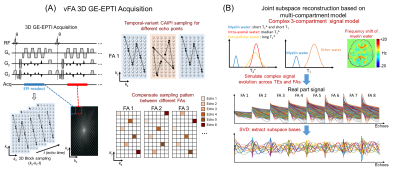
Methods
As shown in Figure 1A, each FA dataset is acquired by 3D gradient-echo EPTI, which acquires multi-echo signals with different ky-kz encodings in k-t space. In 3D GE-EPTI, an EPTI readout is used to cover a small ky-kz block after each RF excitation and after hundreds of TR, high-resolution k-t data can be acquired. The temporal-variant k-t CAIPI pattern is used here, whose improved performance has been validated previously5. Variable flip angle datasets are acquired with complementary k-t encoding (k-t vFA CAIPI) to further improve the accuracy in the joint reconstruction. Since the T2* value of myelin water is very short, non-selective excitation pulse is used to reduce the minimum echo time (TE).The extended complex 3-compartment model (myelin water, intra-axonal water and extracellular water) including T1 effects7 is used here, where each compartment has an exponential T2* decay with different decay rates and different frequency offsets (Figure 1B). Intra-axonal water and extracellular water are assumed to have the same T1 for each voxel, and myelin water has a shorter T1 value. The complex signal evolutions across different FAs and TEs are simulated using 3-compartment model with a wide tissue-parameter range. Temporal bases are then extracted from the simulated signals through PCA, and utilized in the subspace reconstruction. To account for B0 phase differences between different FA datasets (due to e.g. drifts), an initial subspace reconstruction for each FA dataset is performed to extract this phase difference. Using the estimated subspace bases and phase maps, joint subspace reconstruction can be performed to recover the highly-undersampled vFA multi-echo EPTI dataset.
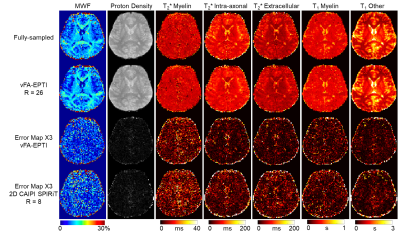
After reconstruction, multiple quantitative parameters are estimated using the 3-compartment model7, including MWF, PD, T1 of myelin water and other water, T2* of myelin, intra-axonal and extracellular water.
The following experiments were performed at 3T using a 32-channel coil.
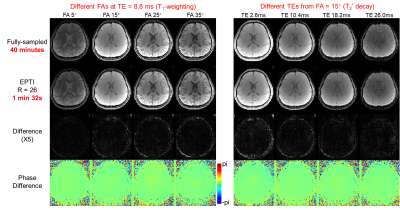
Low-resolution retrospective undersampling validation: Fully-sampled 3D GE-EPTI data were acquired to evaluate the accuracy of the vFA 3D GE-EPTI technique. Acquisition parameters were: FOV=224x174x212 mm3, matrix size=94x72x88, TE range = 1.5ms-32.7ms, echo spacing (ESP)=0.52 ms, number of echoes = 61, TR=47 ms, 8 flip angles are acquired: 5°,10°,15°,20°,25°,30°,35°,40°. The data were retrospectively undersampled in k-t space by 26x to synthesize a vFA 3D-EPTI acquisition, and the reconstructed results were compared with the fully-sampled data.
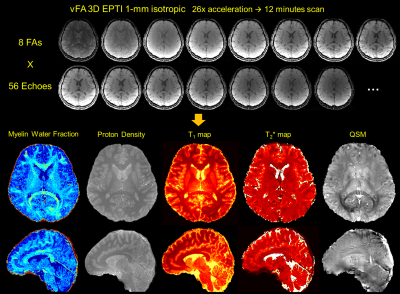
Prospective high-resolution experiment: 1-mm isotropic vFA 3D GE-EPTI data were acquired at an acceleration factor of 26x. Acquisition parameters were: FOV=210x176x210 mm3, matrix size=216x176x210 mm3, TE rang =1.6ms-53.7ms, ESP=0.93 ms, number of echoes = 57, TR=69 ms, the acquisition time of each FA was 90s, the same 8 FAs were acquired, total acquisition time=12 minutes.
Results
Figure 2 shows the comparison between the reconstructed images from fully-sampled acquisition and from undersampled vFA 3D-EPTI at an acceleration factor of 26. VFA 3D-EPTI reduces the scan time from 40 minutes to just 2.5 minutes with minimal image magnitude and phase errors at different FAs and echoes. Figure 3 further demonstrates that the quantitative maps including MWF, PD, multi-compartment T2* and T1 estimated from vFA 3D-EPTI is close to the fully-sampled data, and better than an 8x accelerated dataset with 2D-CAIPI sampling and SPIRiT reconstruction (i.e. at 3.25x lower acceleration). Figure 4 shows the reconstructed images and calculated quantitative maps of 1-mm isotropic vFA-EPTI acquisition, where high-quality myelin water fraction (MWF), proton density (PD), T1, T2* maps, as well as QSM can be extracted from this 12-minute acquisition.Conclusion
The proposed vFA 3D-EPTI method with joint multi-compartment subspace reconstruction achieved high acceleration with good accuracy, providing a powerful technique for fast multi-compartment analysis and microstructural imaging at high spatial resolution. Future work includes extension of the acquisition to other multi-compartment model analysis, and implementation of the technique at 7T to further push the spatial resolution of in-vivo microstructural imaging.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB020613, R01-EB019437, R01-MH116173, P41-EB015896, and U01-EB025162) and the instrumentation Grants (S10-RR023401, S10-RR023043, and S10-RR019307).References
1. Cercignani M, Bouyagoub S. Brain microstructure by multi-modal MRI: Is the whole greater than the sum of its parts? NeuroImage 2018;182:117-127.
2. Does MD. Inferring brain tissue composition and microstructure via MR relaxometry. NeuroImage 2018;182:136-148.
3. Paus T. Imaging microstructure in the living human brain: A viewpoint. NeuroImage 2018;182:3-7.
4. Wang F, Dong Z, Reese TG, Bilgic B, Katherine Manhard M, Chen J, Polimeni JR, Wald LL, Setsompop K. Echo planar time-resolved imaging (EPTI). Magn Reson Med. 2019;81:3599-3615.
5. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo Planar Time-Resolved Imaging (EPTI) with Subspace Reconstruction and Optimized Spatiotemporal Encoding. arXiv preprint arXiv:2019; 1911.00999.
6. Wang F, Dong Z, Reese TG, Wald LL, Setsompop K. 3D-EPTI for Ultra-fast Multi-contrast and Quantitative Imaging. In Proc Intl Soc Mag Reson Med. 2019; Montreal. p 944.
7. Chan KS, Marques JP. Brain tissue multi-compartment relaxometry - An improved method for in vivo myelin water imaging. In Proc Intl Soc Mag Reson Med. 2019; Montreal. p 421.
Figures