0515
Simultaneous Multiple Resonance Frequency (SMURF) imaging: Fat-water imaging using multi-band principles
Beata Bachrata1,2,3, Bernhard Strasser1,2,4, Wolfgang Bogner1,2, Albrecht Ingo Schmid1,5, Siegfried Trattnig1,2,3, and Simon Daniel Robinson1,2,6,7
1High Field MR Centre, Medical University of Vienna, Vienna, Austria, 2Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 3Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 4Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 5Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 6Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 7Department of Neurology, Medical University of Graz, Graz, Austria
1High Field MR Centre, Medical University of Vienna, Vienna, Austria, 2Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 3Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 4Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 5Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 6Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 7Department of Neurology, Medical University of Graz, Graz, Austria
Synopsis
Imaging of body regions containing both water-based and fat-based structures is affected by artefacts arising from the chemical shift difference between water and fat. Recently, a single-echo water-fat separation technique was proposed which used multi-band principles to generate separate water and fat images as well as chemical shift-corrected, recombined water-fat images. We demonstrate the performance of gradient-echo and turbo spin-echo variants of this approach in the knee, breasts and abdomen. The separation of water and fat was similar to or better than with current state-of-the-art techniques and chemical shift effects were fully eliminated in recombined water-fat images.
Introduction
The circa 3.5 ppm1 chemical shift difference between water and fat, gives rise to image artefacts, making the assessment of overlapping water-based and fat-based structures difficult. Firstly, it leads to Type 1 chemical shift artefact; displacement of fat relative to water along the frequency‑encoding direction, resulting in an overlap of their signals, as well as signal voids. Secondly, in gradient-echo imaging, it leads to an echo-time dependent phase disparity between fat and water, resulting in destructive interference if the signals are not acquired at in-phase echo-times; the so-called Type 2 chemical shift artefact.The Dixon approach2 is the only method which simultaneously generates separate images of water and fat. It requires a multi‑echo acquisition which prolongs the acquisition time, and high receiver bandwidth, resulting in poor image SNR. In addition, Dixon images often contain water‑fat swaps which may lead to image misinterpretation.
A proof-of-principle implementation of a single-echo gradient-echo water-fat separation method3 has been recently presented. In this approach, which we refer to as Simultaneous Multiple Resonance Frequency (SMURF) imaging, multi-band pulses4 simultaneously but separately excite fat and water and CAIPIRINHA5,6 with parallel imaging reconstruction7,8 separate the corresponding signals. The resulting water and fat images are either evaluated separately or the fat image is corrected for chemical shift displacement and phase discrepancy and recombined with the water signal. This generates a water-fat image similar to a conventional image obtained using broadband acquisition, but with chemical shift effects fully eliminated9.
Here, we demonstrate the performance of optimized gradient-echo (GRE) and turbo spin-echo (TSE) variants of the SMURF approach in a number of body regions and compare the water-fat separation quality achieved with current state‑of‑art water‑fat separation and suppression techniques; i.e. the Dixon method, and with separate acquisitions using fat- and water-saturation.
Methods
Images of one knee of 3 volunteers, the abdominal region of 3 volunteers and both breasts of 3 volunteers were acquired using a 3T Siemens PRISMA scanner. Sagittal 2D TSE SMURF knee images were acquired with anterior‑posterior phase‑encoding direction, FOV=180x180mm, resolution=0.47x0.47x3mm, 33 slices, 10% slice gap, TE=20.8ms, TR=3000ms, rBW/pixel=145Hz and echo train length=3. Transversal 2D GRE SMURF breasts and abdominal images were acquired in a single breathhold. For the breasts, FOV=320x320mm, resolution=1.0x1.0x3.0mm, 4 slices, 20% slice gap, TE=11.3ms, TR=95ms, rBW/pixel=220Hz, PF=6/8 and phase‑encoding direction left‑right and for the abdomen subject-dependent FOV, resolution=1.2x1.2x3.0mm, 5 slices, 20% slice gap, TE=11.3ms, TR=116ms, rBW/pixel=225Hz and anterior-posterior phase‑encoding direction were used.Low resolution dual‑echo GRE scans were also acquired for B0 field mapping10 and the first echo images were used for water-fat unaliasing with slice-GRAPPA6. Additionally, fat‑saturated (type “strong”11), water‑saturated and/or Dixon images were acquired to allow water-fat separation quality to be compared.
Dixon images of breasts were calculated from 3D dual-echo GRE images acquired with TE={2.27,5.96}ms, TR=13ms, rBW/pixel=445Hz and PF=6/8 using the online sequence (VIBE12) reconstruction. The abdominal Dixon images were calculated from 3D multi‑echo GRE images acquired with TE={1.4,4.2,7.0,9.8,12.6,15.4}ms, TR=17ms and rBW/pixel=1240Hz using the graph‑cut approach13 from the Fat‑water Toolbox14.
The correction of chemical shift displacement of $$$N_{voxels}=\frac{{\Delta}f(water - fat)}{rBW/pixel}$$$ using SMURF was applied in k-space. Since in‑phase acquisition was used for all scans, the correction for phase discrepancy was not necessary.
Results
For all body regions under consideration and all volunteers except one, |ΔB0| < 220 Hz and the SMURF approach generated cleanly separated water and fat images (Figure 2), very similar to the separately acquired fat‑saturated and water‑saturated images (Figure 3). In the breast, SMURF and fat‑saturation methods achieved visibly better fat‑suppression than the 2-point Dixon. In the abdomen, Dixon images of 2 out of 3 volunteers contained severe water‑fat swaps. Dixon images also showed imperfect water‑fat separation in skin and misattribution of the flowing blood signal. Using the SMURF approach, the water and fat signals were misassigned only in a small area of abdominal images of one volunteer, where ΔB0 > 220 Hz (Figure 4). Generally, SMURF achieved at least as good water-fat separation as Dixon. The chemical shift displacement correction removed the misalignment between fat and water and water-fat images completely free of chemical shift effects were generated (Figure 5).Discussion and Conclusion
We have demonstrated the performance of gradient-echo and turbo spin-echo variants of a new water-fat imaging method, Simultaneous Multiple Resonance Frequency (SMURF) imaging, in the knee, breasts and abdomen. The achieved water‑fat separation quality was similar to that using separate acquisitions with fat‑ and water‑saturation and at least as good as with Dixon techniques, despite the approach being based on the assumption of a single fat peak. Unlike Dixon, SMURF requires only a single-echo acquisition and uses robust reconstruction. In addition to separate water-fat imaging, SMURF allows the generation of chemical shift effects-free water‑fat images, removing the restrictions on receiver bandwidths and, in GRE imaging, echo-times. Similar to most of the other water-fat separation/suppression techniques, SMURF requires ΔB0 < 1/2 of the chemical shift difference and the use of generally long, spectrally-selective pulses. In this study, 16.24ms Shinar-Le-Roux15,16 pulses were used, however, shorter pulses could be developed using optimal control17 or VERSE18 approaches. Although the required field homogeneity was satisfied in this study in almost all cases, SMURF could be made viable even where the shim does not meet this requirement19.Acknowledgements
This study was funded by the Austrian Science Fund (FWF) project 31452. SR was additionally supported by the Marie Skłodowska-Curie Action MS-fMRI-QSM 794298. The financial support by the Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and Development is gratefully acknowledged.References
- Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res. 2008;49(9):2055-2062. doi:10.1194/jlr.D800010-JLR200
- Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189-194. doi:10.1148/radiology.153.1.6089263
- Bachrata B, Strasser B, Schmid AI, Bogner W, Trattnig S, Robinson SD. Eliminating chemical shift artefact using simultaneous, separate water and fat excitation combined with CAIPIRINHA. In: Proceedings of the 27th Annual Meeting of ISMRM. Montreal, Quebec, Canada, 2019. p. 4005.
- Müller S. Multifrequency selective rf pulses for multislice MR imaging. Magn Reson Med. 1988;6(3):364-371.
- Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53(3):684-691. doi:10.1002/mrm.20401
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-Controlled Aliasing in Parallel Imaging (blipped-CAIPI) for simultaneous multi-slice EPI with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224. doi:10.1002/mrm.23097
- Pruessmann K, Weiger M, Scheidegger M, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952-962.
- Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47(6):1202-1210.
- Yu H, Reeder SB, Shimakawa A, Gold GE, Pelc NJ, Brittain JH. Implementation and Noise Analysis of Chemical Shift Correction for Fast Spin Echo Dixon Imaging. In: Proceedings of the 12th Annual Meeting of ISMRM. Kyoto, Japan, 2004. p. 2686; :1.
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65-73.
- Optimization of chemical shift selective suppression of fat - Kuroda - 1998 - Magnetic Resonance in Medicine - Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1002/mrm.1910400402. Accessed June 9, 2019.
- Rofsky NM, Lee VS, Laub G, et al. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999;212(3):876-884. doi:10.1148/radiology.212.3.r99se34876
- Hernando D, Kellman P, Haldar JP, Liang Z-P. Robust Water/Fat Separation in the Presence of Large Field Inhomogeneities Using a Graph Cut Algorithm. Magn Reson Med. 2010;63(1):79-90. doi:10.1002/mrm.22177
- ISMRM Fat-water toolbox. ISMRM fat-water separation workshop 2012. “http://www.ismrm.org/workshops/FatWater12/.”
- Shinnar M, Eleff S, Subramanian H, Leigh JS. The synthesis of pulse sequences yielding arbitrary magnetization vectors. Magn Reson Med. 1989;12(1):74-80.
- Le Roux P. Exact synthesis of radio frequency waveforms. January 1988.
- Conolly S, Nishimura D, Macovski A. Optimal Control Solutions to the Magnetic Resonance Selective Excitation Problem. IEEE Trans Med Imaging. 1986;5(2):106-115. doi:10.1109/TMI.1986.4307754
- Hargreaves BA, Cunningham CH, Nishimura DG, Conolly SM. Variable-rate selective excitation for rapid MRI sequences. Magn Reson Med. 2004;52(3):590-597. doi:10.1002/mrm.20168
- Yang C, Deng W, Stenger VA. Simple Analytical Dual-Band Spectral-Spatial RF Pulses for B1+ and Susceptibility Artifact Reduction in Gradient Echo MRI. Magn Reson Med. 2011;65(2):370-376. doi:10.1002/mrm.22725
Figures
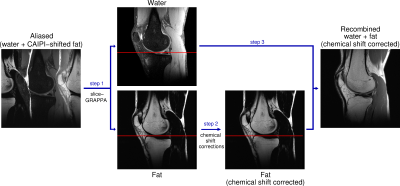
Figure 1: Processing
pipeline of chemical shift corrections using Simultaneous Multiple Resonance Frequency (SMURF) imaging. Overlapping water and CAIPIRINHA‑shifted fat images are
unaliased using slice‑GRAPPA (Step 1). The fat image is corrected for
chemical shift‑related phase evolution (for gradient-echo acquisitions) and
shifted to reverse chemical shift displacement (Step 2) – see the
positions of fat relative to the red reference line. Recombination with the
water image (Step 3) generates a water‑fat image free of chemical shift
effects.
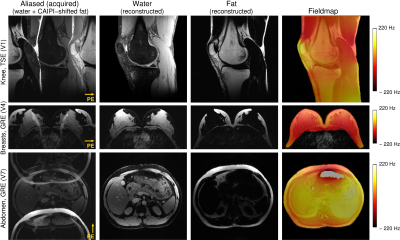
Figure 2: Excitation selectivity and
unaliasing quality of Simultaneous Multiple Resonance Frequency (SMURF) imaging demonstrated for
one exemplary volunteer for each body region under consideration. From the
aliased water and CAIPIRINHA‑shifted fat images, separate images of water and fat are reconstructed. The field inhomogeneity is lower than 220 Hz for all cases,
fulfilling the SMURF requirement. Note the clean water-fat separation without
any cross-excitation or visible residual aliasing artefacts. (The unaliased images were
rescaled for improved visibility.)
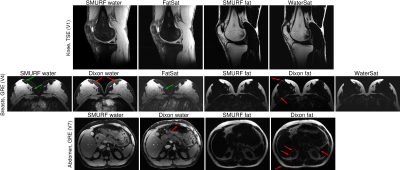
Figure 3: Comparison of SMURF images of water and fat with Dixon images and fat‑saturated (FatSat) and water‑saturated (WaterSat)
images. The knee SMURF images are similar to the separately
acquired FatSat and WaterSat images. In breasts, Dixon water(fat) images show
visibly increased residual fat(water) signal (red arrows), obscuring the
visibility of small water regions surrounded by fat (green arrows). While the abdominal Dixon
images show imperfect water-fat separation over several areas, the SMURF images
show clean separation without any indication of cross-excitation.
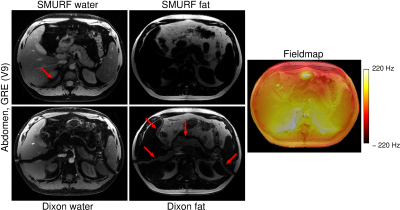
Figure
4: Comparison
of SMURF and Dixon water-fat separation quality in the abdomen with |ΔB0| > ½
of the water-fat chemical shift difference (i.e. 220 Hz). In the SMURF images, the
water and fat signals are misassigned in a small area where the field offset
was greater than 220 Hz.
Dixon images show severe water-fat swaps, extending to areas with smaller field
offsets. The flowing blood signal is also misattributed
to the fat signal in Dixon images.
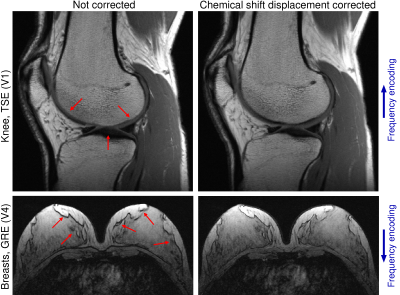
Figure 5: Comparison of not corrected and
chemical shift displacement corrected recombined SMURF water‑fat images. The
correction removes the misalignment between the water and fat (red arrows),
allowing easier assessment of the size and relation between the water-based and
fat-based structures.