0514
Deep Learning-based Quantification of Proton Density Fat Fraction and R2* in the Liver1Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Medicine, University of Wisconsin-Madison, Madison, WI, United States, 6Electrical and Computer Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Multi-echo chemical shift-encoded (CSE)-MRI techniques enable liver PDFF and R2* quantification, which enable staging and treatment monitoring of liver fat and iron content, respectively. However, the common requirement of breath-holding in CSE-MRI acquisitions is challenging for many patients. Furthermore, the required specialized multi-echo acquisition and reconstruction are not available in all scanners. In this work, we assessed the accuracy of deep learning (DL)-based PDFF and R2* quantification using reduced numbers of echoes. Preliminary results demonstrate the potential of this approach and suggest that at least four echoes are needed for quantifying PDFF and R2* at 1.5T and 3.0T.
INTRODUCTION
Abnormal accumulation of intracellular triglycerides in the liver is the earliest, hallmark feature of non-alcoholic fatty liver disease (NAFLD), which is the most common cause of diffuse liver disease, afflicting an estimated 100 million people in the US alone1. Further, hepatic iron overload is common in patients with genetic hemochromatosis or transfusional hemosideroris. MR-based chemical shift-encoded (CSE) techniques enable non-invasive measurement of the proton density fat fraction (PDFF) and R2*, an established biomarker of tissue triglyceride concentration2 and liver iron concentration3, respectively. Images acquired at multiple echo times are commonly obtained in a single breath-hold acquisition (e.g., 20 seconds) for simultaneous mapping of PDFF and R2*4. However, many patients are unable to sustain such prolonged breath-hold, leading to image artifacts and inaccurate quantification. Moreover, the specialized CSE-MRI acquisitions and reconstructions required to correct for all relevant confounding factors are not accessible across imaging sites, particularly in non-academic sites. Convolutional neural network (CNN) based approaches have the potential to overcome these challenges by quantifying PDFF and R2* with a reduced number of echoes. Previous studies5-6 have shown the feasibility of deep learning (DL)-based PDFF quantification using reduced echoes at 1.5T. The purpose of this work was to preliminarily assess the accuracy of DL-based PDFF and R2*. quantification with different numbers of echoes at both 1.5T and 3.0T.METHODS
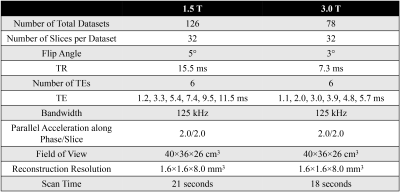
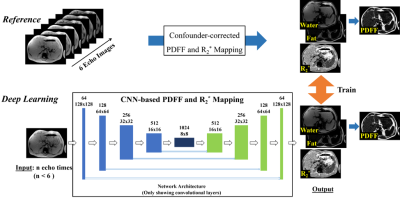
MRI Data: 126 datasets of subjects imaged at 1.5T (Optima MR 450w, GE Healthcare) and 78 datasets of subjects imaged at 3.0T (MR750, GE Healthcare) in two HIPAA compliant and IRB-approved studies were retrospectively analyzed. A breath-hold 3D 6-echo spoiled gradient-echo (SGRE) acquisition (Table 1) was obtained in each subject. The reference proton density-weighted water image, fat image and R2* map were reconstructed from the magnitude images of each SGRE dataset by using a confounder-corrected chemical shift-encoded reconstruction7.Network architecture: A U-Net CNN8 was used with multi-channel input (magnitude multi-echo images) and three output maps (i.e. water image, fat image and R2* map). This CNN architecture was trained several times using magnitude images of the first n (n≤6) echoes as input. The network architecture is illustrated in Figure 1. The training loss function was defined as the L1-norm between the reference maps and output maps of the U-Net. The network was trained on NVIDIA Titan Xp using the Adam optimizer with learning rate = 0.00001, batch size = 3, and 200 epochs.
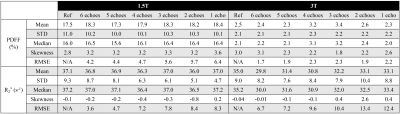
Data Analysis: PDFF maps were calculated from the estimated water and fat images. The mean, standard deviation, median, skewness and root-mean-square-error (RMSE) of the quantitative parameters (PDFF and R2*) in the liver of the test datasets were calculated and compared to the reference.
RESULTS
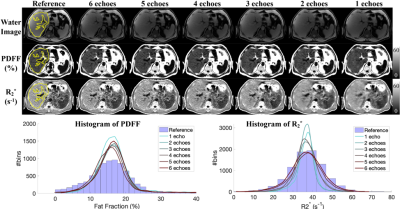
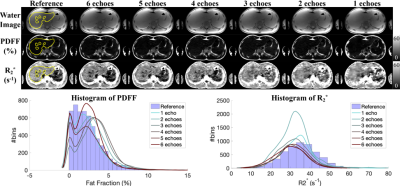
Figures 2 and 3 show the test results of two different subjects at 1.5T and 3.0T, respectively, including water images, PDFF and R2* maps estimated using CSE-based reconstruction (reference in the 1st column) and DL-based reconstruction using all 6 echo images (the 2nd column) and reduced echo images (1-5 echoes in the 3rd to 7th columns). At both 1.5T and 3.0T, DL-based reconstruction is capable of separating water and fat with 0-100% dynamic range of PDFF from magnitude images, while the dynamic range of PDFF achievable in conventional magnitude-based reconstruction is 0-50%. The liver contour excluding vessels is delineated with yellow lines on the reference images in Figures 2 and 3 and the quantitative measurements are listed in Table 2. In these two test datasets, the liver PDFF and R2* measurements are accurate in DL-based reconstruction with reduced echoes, indicated by the consistent estimated mean and median values (Table 2). The sharpness of tissue boundaries (e.g., liver tissue and vessels) is maintained in DL-based reconstruction of PDFF and R2* maps using all 6- or 5-echo images.The sharpness is degraded in liver tissues and small vessels, especially in the R2* map using 4-echo images. Blurring was observed with both PDFF and R2* maps reconstructed using 1-3 echo images. At 1.5T in Figure 2, the histograms of the PDFF of the contoured liver tissues are narrower compared in DL-based reconstruction compared to the reference. The histograms of the liver R2* in DL-based reconstruction using all 6- or 5-echoes closely match the reference but become narrower using 1-4 echoes. At 3.0T (Figure 3), the histograms of the liver R2* in DL-based reconstruction are shifted towards a smaller R2* (negative bias) compared to the reference.
DISCUSSION AND CONCLUSION
In this study, we have assessed the feasibility and accuracy of DL-based PDFF quantification using different numbers of echoes at both 1.5T and 3.0T. Our preliminary results suggested that accurate PDFF measurements can be achieved using at least 4 echoes. Image quality including the sharpness of tissue boundaries and the accuracy of R2* maps was degraded in reconstructions that used 3 or fewer echoes. This is likely due to the failure of capturing the slow T2* decay of liver tissue in subjects with a R2* of 30-40s-1 at 1.5T and 3.0T at short echo times. Future work will include enriched training datasets that include a larger range of PDFF and R2* values, and comparison of DL-reconstruction using different echo time combinations.Acknowledgements
The authors acknowledge support from the NIH (grants R01-DK117354, R01-DK100651, K24-DK102595 and U01-HD087216) as well as GE Healthcare who provides research support to the University of Wisconsin-Madison. The authors also gratefully thank for Chuhan Gao, Xihui Liu, Huiwen Luo and Kai Wang for helpful discussions. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Rinella ME. Nonalcoholic Fatty Liver Disease: A Systematic Review. JAMA. 2015;313(22):2263-2273.
2. Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: A standardized mr-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5):1011-1014.
3. Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460-1465.4.
4. Hines CDG, Frydrychowicz A, Hamilton G, et al. T1 independent, T2* corrected chemical shift based fat–water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging. 2011;33(4):873-881.
5. Andersson J, Ahlström H, Kullberg J. Separation of water and fat signal in whole‐body gradient echo scans using convolutional neural networks. Magn Reson Med. 2019;82(3):1177-1186.6.
6. Goldfarb JW, Craft J, Cao JJ. Water–fat separation and parameter mapping in cardiac MRI via deep learning with a convolutional neural network. J Magn Reson Imaging. 2019.7.
7. Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34(4):729-749.
8. Liu F, Feng L, Kijowski R. MANTIS: Model-Augmented Neural neTwork with Incoherent k-space Sampling for efficient MR parameter mapping. Magn Reson Med. 2019;82(1):174-188.
Figures