0511
Transient-State Inhomogeneous Magnetisation Transfer: Towards Magnetisation Transfer Fingerprinting
Daniel J. West1, Gastao Cruz1, Olivier Jaubert1, Rui P. A. G. Teixeira1,2, Torben Schneider3, Jacques-Donald Tournier1,2, Jo Hajnal1,2, Claudia Prieto1, and Shaihan J. Malik1,2
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for The Developing Brain, King's College London, London, United Kingdom, 3Philips Healthcare, Guildford, United Kingdom
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for The Developing Brain, King's College London, London, United Kingdom, 3Philips Healthcare, Guildford, United Kingdom
Synopsis
Inhomogeneous magnetisation transfer (ihMT) is a contrast mechanism that has shown high specificity towards myelinated tissue. Contrast is typically generated using sequences comprising a preparation phase with several RF saturation pulses, followed by multiple readout periods for measurement. Here, we present a transient acquisition scheme that alternates between periods of multi-band and single-band RF pulses, to efficiently generate ihMT contrast during a single data acquisition. Since signal is transiently varying throughout, we use a dictionary-based low-rank inversion reconstruction method originally proposed for magnetic resonance fingerprinting. Simulation, phantom and human in-vivo experiments are included.
Introduction
The recently developed inhomogeneous magnetisation transfer (ihMT) contrast mechanism has shown a high specificity towards myelinated tissue1,2. ihMT contrast generation relies on using single- or dual-frequency off-resonance radiofrequency (RF) saturation pulses that are usually applied during preparation phases prior to readout periods for measurement3. We have previously developed non-selective multi-band (MB) RF pulses that: (1) control magnetisation transfer (MT) effects in variable flip angle relaxometry4 and (2) generate ihMT contrast in a steady-state framework, through simultaneous free water excitation and semisolid saturation5.Building upon these works and the fact that others have shown that ihMT contrast can be increased by reducing duty cycle6, we now consider a transient-state ihMT (TS-ihMT) sequence implementation. Figure 1 shows the sequence structure, with alternating blocks of MB and single-band (1B) pulses. The on-resonance bands of all pulses are identical, so the free water flip angle remains constant throughout; a tissue with no MT or ihMT effect would give constant signal. However, tissues with MT effects will respond transiently, as sketched in Figure 1.
Methods
The sequence in Figure 1 forms a cyclic steady-state that repeats many times: one cycle (2B-1B-3B-1B) takes ~6 seconds for TR=5ms. ihMT contrast is related to the signal difference between 2B and 3B pulse periods. We optimise properties of this pulse cycle for efficiency of ihMT contrast generation (per square root of acquisition time). To fully characterise the transient evolution of the sequence, ~1000 images would be required, which is infeasible. However, temporal redundancies in the evolution of magnetisation can be exploited with a temporal subspace model reconstruction. To do this we employ a magnetic resonance fingerprinting (MRF) style acquisition scheme using a radial k-space trajectory with low-rank inversion (LRI) reconstruction technique from Assländer et al.7Dictionary Creation: A dictionary of expected signal evolutions was created for a range of model parameters (both free-pool and MT/ihMT parameters) for balanced SSFP and SPGR variants of the sequence. For bSSFP B0 variation was additionally included. Singular value decomposition (SVD) suggests that the dictionary can be well-characterised by few singular components.
Data Acquisition: A 3D tiny golden angle radial8, stack-of-stars k-space trajectory with one spoke per RF pulse was implemented for both balanced and spoiled readouts. Acquisition time was variable but used an integer number (one or two) loops over the full cycle of all RF pulses (1200 pulses) per slice-encode position.
Image Reconstruction: Based on a low-rank representation of the dictionary, LRI replaces the reconstruction of the entire transient sequence (~1000 images) by a reconstruction of six singular value images. Rather than perform dictionary matching, the time-series data were processed to yield MT metrics, where min and max indicate timepoints corresponding to the minimum or maximum signals during these periods.
$$(1)ihMTR = \frac{|min(S_{2B})-min(S_{3B})|}{max(S_{1B})}$$ $$(2)MTR = \frac{min(S_{2B})+min(S_{3B})}{2max(S_{1B})}$$
Experiments were conducted on a 1.5T Philips Ingenia MRI system. 3 phantoms were scanned: MnCl2-doped water (0.05mM), bovine serum albumin (BSA) and prolipid 161 (PL161). One healthy male volunteer (age 32) was scanned; sagittal view, isotropic resolution 1.5mm, total scan time of ~25 minutes for a 3D volume.
Results
Optimisation of efficiency requires modification of flip angle (FA), repetition time (TR), RF pulse duration (τ), off-resonance frequency (∆f), nMB and n1B. Figure 2 shows the ihMTR from TS-ihMT compared to a recently published steady-state approach5 (referred to as SS-ihMT for comparison), for WM-like tissue parameters3,6.Single-slice reconstructed phantom images from TS-ihMT are shown in Figure 3, alongside reconstructed signal time courses for central regions-of-interest (ROIs) in each tube, normalised by their mean values. Signals agree well with 1D encoded measurements made without using the dictionary-based LRI.
Figure 4 shows preliminary results from TS-ihMT in-vivo experiments, using both bSSFP and SPGR; Figure 5 shows corresponding singular images used in their reconstruction and the log of singular values of each dictionary to highlight the low-rank nature of the problem.
Discussion
Figure 2 demonstrates that the transient method can generate an approximate 2-fold increase in the peak ihMTR contrast compared to a steady-state approach. Phantom data show that the LRI reconstruction produces time courses that closely match 1D measurements. The phantoms showed expected contrast with no MT or ihMT in water, MT only in BSA and ihMTR of approximately 25% measured in PL161.In-vivo maps show GM-WM contrast as expected in both MTR and ihMTR, and the time profiles are as expected. Although acquisition of this data took ~25 minutes, the cyclic steady-state and LRI reconstruction are flexible and acquisition time can be optimised. The time profiles for CSF show some variability when none is expected, mainly in the spoiled case; this is attributed to residual reconstruction errors which generally affect the ihMTR map from the spoiled data. Potential improvements will include updating the reconstruction to include motion estimation and hardware imperfection, adding unmodelled effects such as diffusion/flow/partial volume to the dictionaries, and considering alternative k-space ordering schemes.
Conclusions
Our results show that the TS-ihMT approach can be used to flexibly measure MT and ihMT contrast. Though we have so far used the reconstructed time course data to create ihMTR/MTR maps, the time profiles contain much richer information and we will explore dictionary matching and related approaches to extracting quantitative tissue parameter estimates.Acknowledgements
This work was funded by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging [EP/L015226/1] and supported by the Wellcome EPSRC Centre for Medical Engineering at King's College London [WT 203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
- Girard, O. M. et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn. Reson. Med. 73, 2111–2121 (2015).
- Ercan, E. et al. Microstructural correlates of 3D steady-state inhomogeneous magnetization transfer (ihMT) in the human brain white matter assessed by myelin water imaging and diffusion tensor imaging. Magn. Reson. Med. 80, 2402–2414 (2018).
- Mchinda, S. et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn. Reson. Med. 79, 2607–2619 (2018).
- A.G. Teixeira, R. P., Malik, S. J. & Hajnal, J. V. Fast quantitative MRI using controlled saturation magnetization transfer. Magn. Reson. Med. 1–14 (2018). doi:10.1002/mrm.27442
- Malik, S. J., Teixeira, R. P. A. G., West, D. J., Wood, T. C. & Hajnal, J. V. Steady‐state imaging with inhomogeneous magnetization transfer contrast using multiband radiofrequency pulses. Magn. Reson. Med. 1–15 (2019). doi:10.1002/mrm.27984
- Varma, G. et al. Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. J. Magn. Reson. 296, 60–71 (2018).
- Assländer, J. et al. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med. 79, 83–96 (2018).
- Wundrak, S. et al. Golden Ratio Sparse MRI Using Tiny Golden Angles. Magn. Reson. Med. 2378, 2372–2378 (2016).
Figures
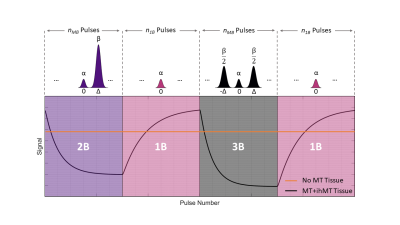
Figure 1: The
proposed acquisition uses a rapid gradient echo sequence (either spoiled or
balanced) alternating between the pulses shown in the top row in blocks; nMB indicates the number of MB pulses in a block, for example. The
on-resonance part of each pulse is identical, so free water (no MT effect)
would give constant signal throughout the acquisition. The switched
off-resonance bands will affect semisolid proton saturation, resulting in
modulation of the free water signal via magnetisation transfer as shown in the
plot. 2B and 3B pulses are alternated to give ihMT contrast.
Figure 2: Table: Optimal parameters for maximising efficiency of ihMT contrast generation
in transient and steady-state sequences. Middle: Signal for the transient sequence, compared with only on-resonance pulses. Bottom:
Simulated ihMT ratio (ihMTR); in the transient-state the peak effect is larger
than in the steady-state, but it evolves over time. Note, the transient phase
at the start of the acquisition is excluded for subsequent data analysis; the
acquisition is repeated for very many cycles, so this transient period makes up
a negligible part of the overall data.
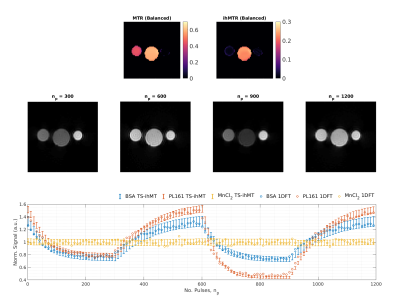
Figure 3: Top:
ihMTR and MTR maps calculated from a balanced acquisition. From left to right,
tubes are BSA, PL161 and MnCl2-doped water. As expected, water shows
no MTR or ihMTR; BSA only MTR; and PL161 ihMTR as well. Middle: Single-slice reconstructed phantom images at different
timepoints from a spoiled acquisition. Bottom:
Bars represent corresponding reconstructed time courses (and their standard
deviation) from a ROI in each tube and circles are from a non-phase encoded
experiment (‘1DFT’) to accurately measure time-response without using the LRI
reconstruction.
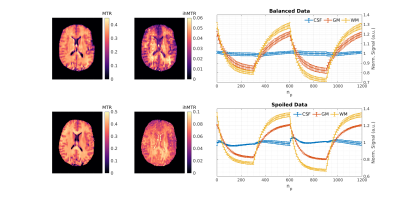
Figure 4: Single-slice
ihMTR and MTR maps from a healthy volunteer, obtained
using Equations 1 and 2. Time courses are also shown for cerebrospinal fluid
(CSF), grey matter (GM) and WM ROIs to demonstrate obtainable ihMT contrast.
Spoiled data was acquired using: FA = 6.2˚, τ =
2.5ms, ∆f =
8.28kHz, B1,rms = 4.0μT, nMB = 300, n1B = 300, TR = 5.9ms and balanced
data was acquired using: FA = 29.5˚, τ =
2.5ms, ∆f =
8.0kHz, B1,rms = 4.0μT, nMB = 300, n1B = 300, TR = 5.3ms. Residual
aliasing is apparent in the spoiled ihMTR map and can also be seen in the more
variable CSF signal.
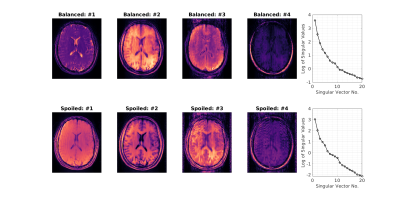
Figure 5: Singular
images used in the reconstruction of balanced and spoiled in-vivo data. In each
case, >95% of the variability can be described by the first two components
and beyond the fourth, singular images show little structure.