0508
Towards clinical CEST-MRF: whole brain snapshot CEST MR Fingerprinting at 3T using spin-lock saturation and a centric 3D-EPI readout1Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 3German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 4Department of Physics and Astronomy, University of Bonn, Bonn, Germany, 5Department of Biomedical Magnetic Resonance, Eberhard Karls University Tuebingen, Tuebingen, Germany, 6Department of Neuroradiology, University Hospital Erlangen, Erlangen, Germany
Synopsis
Quantitative CEST imaging is still not applied in clinical routine, as both quantification and whole brain coverage require usually long scan times. In this work, we present a CEST-MRF protocol using spin-lock saturation pulses and a fast 3D-EPI readout with whole brain coverage. This enables a fast generation of quantitative amide proton concentration maps of the entire brain at a clinical scanner.
Introduction
Quantitative CEST imaging is still not applied in clinical routine, as quantification requires repeated scans, and clinical evaluation in the brain requires whole brain coverage. Both prolongs the MR signal acquisition. In terms of repeated acquisition, an efficient CEST MR-Fingerprinting approach was presented recently as a tool for rapid, quantitative CEST imaging1,2. By combining CEST-MRF with a fast 3D snapshot readout, we propose a solution for both short scan time and whole brain coverage. The easy to use 3D snapshot CEST-MRF approach allows then for fast and robust quantitative CEST contrast generation at clinical field strengths.Methods
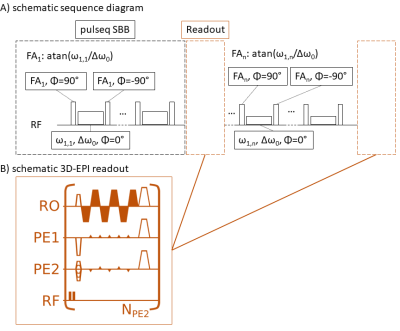
Imaging was performed at a 3T clinical scanner (Siemens Prisma) using a vendors 64 channel head/neck coil on a healthy subject after obtained written informed consent. A centric reordered 3D-EPI readout3 (cf. Fig. 1b, FOV: 256x224x156mm³, CAIPIRINHA=1x6shift=2; partial Fourier=6/8x1; BW=1930Hz/pixel; TE=11ms; EPI-factor=32; NPE2=45 binominal-11 water excitations per volume; FA=15°, 1.8mm3 isotropic, tRO=1.2s) was used for imaging after the saturation phase.The amide sensitive saturation schedule consisted of 31 repetitions of a spin lock saturation train (13x100 ms, 50% duty-cycle) at 3.5 ppm, each followed by a 3D-EPI readout. Across repetitions, saturation power was varied between 0-4 µT. For a flexible and rapid implementation of the MRF saturation schedule, the pulseq4 sequence was adapted to be used as a sequence building block. The sequence diagram is shown in Figure 1. For masking, SPM was used to generate a GM and WM segment from a T1 map that was obtained with a saturation recovery sequence.
For dictionary generation, the applied sequence was simulated using Bloch-McConnell equations for approx. 12.5 M different combinations of water T1 and T2, as well as amide concentration fractions (f) and exchange rates (k). The dictionary generation and matching procedure using the dot product metric was performed as recently presented2.
Results
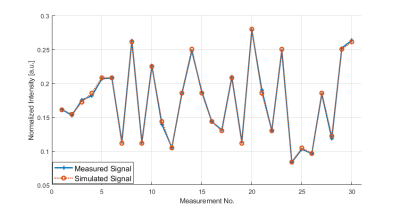
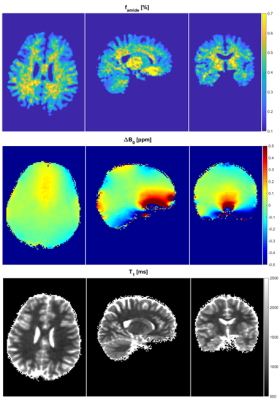
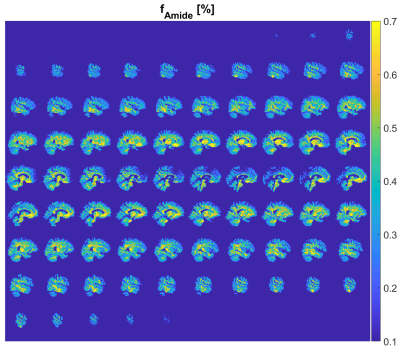
Dictionary generation took approx. 11.5 hours (Intel XEON W-2145 CPU, 32 GB RAM) and another 5.5 hours were needed for the voxel-wise matching procedure of the acquired trajectories to the dictionary. An example matching trajectory for a WM voxel is shown in Figure 2. Resulting amide concentration maps masked with the GM/WM segment are shown in Figure 3 and 4. The concentration maps (Fig. 3, first row) show similar contrast as in previous animal experiments1. In addition the gray/white matter contrast resembles other sophisticated APT contrast maps as e.g. AREX5 which should be proportional to k·f. The contrast shows a small correlation with the field inhomogeneity map (Fig. 3, last row). Except for this correlation, the matched values are spatially homogeneous over the whole brain (Fig. 4), which proves the robustness of the method. In addition to amide proton fraction maps, amide proton exchange rate maps can be generated (data not shown herein).Discussion
With an easy adaptable saturation block and a rapid whole brain 3D-EPI MRI acquisition, we introduce a powerful tool for not only CEST-MRF, but also CEST imaging in general. First results are promising and a quantitative amide proton concentration map was acquired in only 2:47 min. In this study, dictionary matching was the slowing factor, especially due to the 3D image data. In the future, neural networks can be used to map directly between acquired images and dictionaries6, which will speed up calculation and enable denoising features. In addition, dictionaries or neural networks incorporating both B0 and B1 inhomogeneity would further stabilize the method. Additional improvement is expected by adding a semi-solid MT pool to the simulation of the dictionary.Conclusion
By using a pulseq saturation block and a fast 3D-EPI readout for image acquisition, we were able to generate quantitative, whole brain amide concentration maps within a measurement time below 3 minutes. This enables application of CEST-MRF in a clinical environment.Acknowledgements
The financial support of the Max Planck Society, German Research Foundation (DFG; grant ZA 814/2‐1, support to K.H.), and European Union's Horizon 2020 research and innovation program (Grant Agreement No. 667510, support to M.Z.) is gratefully acknowledged. O.P. acknowledges funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No. 836752 (OncoViroMRI).References
1: Cohen, O, Huang, S, McMahon, MT, Rosen, MS, Farrar, CT. Rapid and quantitative chemical exchange saturation transfer (CEST) imaging with magnetic resonance fingerprinting (MRF). Magn Reson Med. 2018; 80: 2449– 2463. https://doi.org/10.1002/mrm.27221
2: Perlman, O, Herz, K, Zaiss, M, Cohen, O, Rosen, MS, Farrar, CT. CEST MR‐Fingerprinting: Practical considerations and insights for acquisition schedule design and improved reconstruction. Magn Reson Med. 2020; 83: 462– 478. https://doi.org/10.1002/mrm.27937
3: Akbey, S, Ehses, P, Stirnberg, R, Zaiss, M, Stöcker, T. Whole‐brain snapshot CEST imaging at 7 T using 3D‐EPI. Magn Reson Med. 2019; 82: 1741– 1752. https://doi.org/10.1002/mrm.27866
4: Layton, K. J., Kroboth, S. , Jia, F. , Littin, S. , Yu, H. , Leupold, J. , Nielsen, J. , Stöcker, T. and Zaitsev, M. (2017), Pulseq: A rapid and hardware‐independent pulse sequence prototyping framework. Magn. Reson. Med., 77: 1544-1552. doi:10.1002/mrm.26235
5: Goerke, S, Soehngen, Y, Deshmane, A, et al. Relaxation‐compensated APT and rNOE CEST‐MRI of human brain tumors at 3 T. Magn Reson Med. 2019; 82: 622– 632. https://doi.org/10.1002/mrm.27751
6: Cohen, O, Zhu, B, Rosen, MS. MR fingerprinting Deep RecOnstruction NEtwork (DRONE). Magn Reson Med. 2018; 80: 885– 894. https://doi.org/10.1002/mrm.27198
Figures