0506
High-resolution pH imaging with ratiometric CEST and BIRDS using dual paramagnetic DOTA–tetraglycinate agents1(1)Magnetic Resonance Research Center (MRRC), Yale University, New Haven, CT, United States, 2(2)Department of Diagnostic Radiology, Yale University, New Haven, CT, United States, 3Core Center for Quantitative Neuroscience with Magnetic Resonance (QNMR), Yale University, New Haven, CT, United States
Synopsis
Chemical Exchange
Saturation Transfer (CEST) and Biosensor
Imaging of Redundant Deviation in Shifts (BIRDS) biosensing methods differ respectively by detecting
exchangeable and non-exchangeable protons on the agent. Given that CEST
and BIRDS properties observed from the same paramagnetic agent are
complimentary, we describe a novel approach for high-resolution pH imaging
using dual agents of europium and thulium complexed with DOTA-tetraglycinate.
In vitro results test the hypothesis that ratiometric paraCEST attributes are
conserved when temperature from paraBIRDS is detected simultaneously, enabling absolute
pH imaging. In vivo results in glioblastoma demonstrate feasibility of this
dual paraCEST-paraBIRDS biosensing method for high-resolution pH imaging.
Introduction
MRI contrast can be improved by enhancing relaxation with paramagnetic agents(1,2).Despite great success of the gadolinium-based-agents in clinical MRI, the generated contrast is always “on” when the agent is present, and thus the approach lacks sensitivity and specificity. A novel class of responsive agents become available with chemical exchange saturation transfer (CEST) MRI(3,4), where exchange of water protons and hydroxyl/amine/amide protons are intrinsically pH and temperature dependent. The development of many paramagnetic CEST (paraCEST) agents has proceeded with the aim of pushing the chemical shift of exchangeable protons further away from the bulk water proton signal, thereby reducing direct saturation of water protons. Today many lanthanide-based paraCEST agents are used for molecular imaging(5,6). Although ratiometric paraCEST has shown some promise for molecular imaging, CEST imaging with multiple agents can also be improved accounting for the ambient conditions (e.g., temperature) to tease out the fact that any CEST contrast is intrinsically pH dependent. In addition to the exchangeable protons of the paraCEST agents, the nonexchangeable proton resonances emanating from the same paramagnetic agent are also utilized by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS)(7,8)for biosensing which is a 3D chemical shift imaging (CSI) method. Various paramagnetic BIRDS (paraBIRDS) agents have been applied to map temperature and/or pH in vivo(9,10). In the present work, we hypothesize that pH imaging using ratiometric paraCEST can be calibrated by temperature mapping from paraBIRDS, where both signals are emanating from the same two agents in the same voxels.Methods
A series of phantoms of TmDOTA-(gly)4- and EuDOTA-(gly)4- and their mixture (Macrocyclics, Dallas, TX) were prepared using 10 mM phosphate buffer at various pH values (6.4-7.9) with 10% D2O for BIRDS and CEST experiments. All NMR characterization experiments were acquired with an 11.7 T vertical magnet (Bruker, Billerica, MA). BIRDS properties for individual and the mixture samples were recorded by acquiring proton spectra at various temperatures (27-40 °C). CEST experiments were collected with 4s continuous wave saturation pulse (16.7µT) over a range of frequencies (± 100 ppm). The same phantoms were used for BIRDS and CEST imaging on a 11.7T horizontal bore spectrometer using a volume Birdcage coil (4 cm), field of view 32 × 32 mm. CSI experiments were acquired using 25 x 25 encoding steps, TR=10 ms. A 600 µs Gaussian pulse was used for excitation. CEST images were acquired using a spin-echo imaging sequence with image matrix of 64 × 64. The temperature was controlled by circulating hot air in the magnet bore, monitored by thermocouple. The same experimental setup described above was used for in vivo study. All animal experimental procedures on rats were approved by the Institutional Animal Care and Use Committee (IACUC). Fischer 344 rats received an implantation of RG2 glioma cells. The tail vein was used for injection of TmDOTA-(gly)4- and EuDOTA-(gly)4- mixture (1mmol/kg/hour). A water-heating blanket was used to control and maintain the body temperature.Results
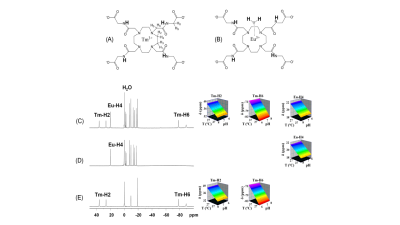
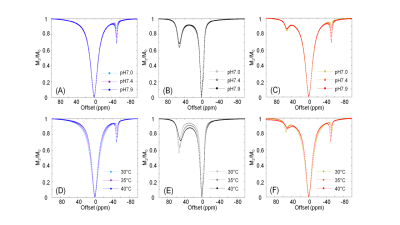
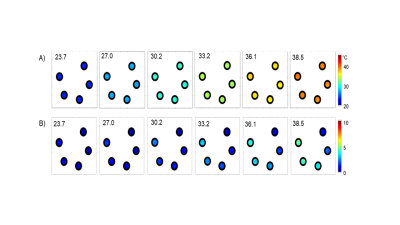
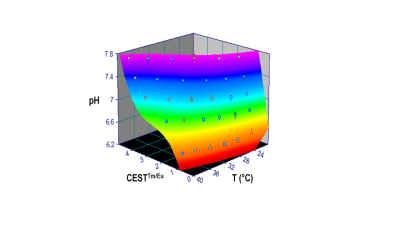
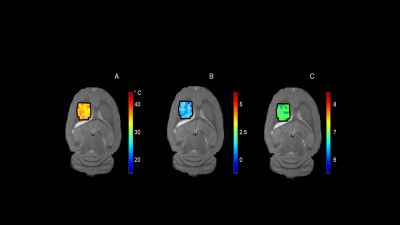
Proton NMR spectra of TmDOTA-(gly)4- and EuDOTA-(gly)4-, and their equivalent mixture show characteristic hyperfine shifts for nonexchangeable proton resonances (Figure 1A-C). As a result, proton resonances Tm-H2 and Tm-H6 in TmDOTA-(gly)4- and Eu-H4 in EuDOTA-(gly)4- are chose for BIRDS characterization (Figure 1D-F). The amide protons in TmDOTA-(gly)4- resonating at -52.5 ppm, while the bound water protons in EuDOTA-(gly)4- resonating at 51.5 ppm at 35 °C are indicated by CEST spectra (Figure 2). To determine the pH, BIRDS was used to read the temperature and the ratio of the CEST intensities between TmDOTA-(gly)4- and EuDOTA-(gly)4- traced pH dependency. Temperature maps from TmDOTA-(gly)4- and EuDOTA-(gly)4- were used to generate the average temperature maps of the mixture (Figure 3A) and CEST maps were used to create the ratiometric CEST maps in the mixture at different temperatures (Figure 3B). 3D surface plot (Figure 4) for pH determination was then fitted as function of temperature (T) and paraCEST ratio (R): pH=a+bR+cT+dR2+eT2+fRT+gR3+hT3+iRT2+jR2T In vivo experiment for rat’s brain tumor is shown on Figure 5A-C. Temperature mapping with BIRDS show an average temperature of 35.6±0.8°C inside the tumor, while ratiometric paraCEST imaging readout show a ratio of 2.0±0.2. The calculated pH map from ratiometric paraCEST calibrated with BIRDS indicate an average pH value of 7.0±0.7 which reflects the physiological microenvironment of solid tumors characterized by extracellular acidity.Discussion
In this study, TmDOTA-(gly)4- and EuDOTA-(gly)4- were used as a model system of multivalent agents to demonstrate ratiometric imaging with paraCEST that is calibrated from temperature readout with paraBIRDS, enabling the pH-dependence of CEST contrast to be extrapolated for quantitative pH values. BIRDS properties of multivalent agents are not perturbed, whereasthe CEST properties are altered. Moreover, the temperature sensitivities in BIRDS can be enhanced in the mixture of two agents because of the increased redundancy of reporting. We show that the ratiometric CEST is temperature dependent, which can be calibrated with BIRDS, where the temperatures measured with BIRDS could be combined with the ratiometric CEST for accurate temperature-independent pH reporting. As we have shown in vivo, pH readouts vary with temperature.Conclusions
High spatial resolution pH maps in rat brain tumors can be obtained using ratiometric paraCEST, with temperature calibrated by paraBIRDS. This paraCEST/paraBIRDS-based temperature-independent pH mapping technique can be used for longitudinal monitoring of therapeutic response in tumors, where temperatures might be different between pathological and non-pathological tissue.Acknowledgements
References
1. Kubicek, V. and E. Toth, Design and Function of Metal Complexes as Contrast Agents in Mri. Advances in Inorganic Chemistry, Vol 61: Metal Ion Controlled Reactivity, 2009. 61: p. 63-129.
2. Viswanathan, S., et al., Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem Rev, 2010. 110(5): p. 2960-3018.
3. Sherry, A.D. and M. Woods, Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng, 2008. 10: p. 391-411.
4. van Zijl, P.C. and N.N. Yadav, Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med, 2011. 65(4): p. 927-48.
5. Evbuomwan, O. M, et al. CEST and PARACEST Agents for Molecular Imaging. In The Chemistry of Molecular Imaging 2014. pp. 225-243.
6. Woods M, et al. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. ChemSocRev (2006).35(6):pp 500-511
7. Coman, D., H.K. Trubel, and F. Hyder, Brain temperature by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS): comparison between TmDOTP5- and TmDOTMA. NMR Biomed, 2010. 23(3): p. 277-85.
8. Coman, D., et al., Brain temperature and pH measured by (1)H chemical shift imaging of a thulium agent. NMR Biomed, 2009. 22(2): p. 229-39.
9. Coman D, et al., Distribution of temperature changes and neurovascular coupling in rat brain following 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") exposure.NMRBIomed,2015.28(10):1257-66.
10. Walsh JJ et al., Dynamic Thermal Mapping of Localized Therapeutic Hypothermia in the Brain.JNeurotrauma, 2019. doi: 10.1089/neu.2019.6485.
Figures