0488
Studying Human Plantar Flexor Muscles at Low-Intensity Exercise by Interleaving Perfusion 1H MRI with Localised 31P MRS1Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Wien, Austria, 2High-Field MR Centre, Medical University of Vienna, Wien, Austria, 3Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Wien, Austria
Synopsis
The purpose of this study is to show the effect of low-intensity exercise on localised 31P MR spectra and 1H images of human muscle. It was determined which PCr depletion is sufficient for quantifying the PCr recovery time constant (τPCr), while incurring minimal pH drop in a predominantly glycolytic muscle. PCr depletion during exercise was measured, PCr recovery was fitted and pH was quantified with dynamic localised 31P MRS, while perfusion and BOLD 1H images were simultaneously acquired in time-resolved measurements. Prolongation of τPCr with acidification was confirmed, while very short τPCr was found with neutral and slightly acidic pH.
Introduction
Exercise/recovery studies of human muscle using 31P MRS have mostly been performed by prescribing a fixed exercise intensity as a fraction of the subjects’ maximum voluntary contraction force (MVC), typically 30 % or more. The purpose is to deplete phosphocreatine (PCr) sufficiently much to measure the time constant of its recovery post-exercise, τPCr. This rate is closely tied to the oxidative ATP production rate, and is hence used to quantify muscle ‘mitochondrial capacity’ 1 – which is straight-forward only if pH at end of exercise (pHee) does not fall by more than 0.1 – 0.2 units due to glycolytic contributions to ATP synthesis. While quite significant PCr depletion (~ 50 %) has been reached without observations of acidification with in partly- or unlocalised MRS 2, more recent experience with muscle-specific 31P MRS localisation reflected pH changes at already relatively low exercise intensities 3,4. A way to avoid acidification is to monitor 31P spectra online and stop exercise upon PCr drop, which may require modifications of the scanner. Short, intense-exercise protocols have been proposed 2, as well as exercising a sufficiently large mass of muscle tissue 5 or exercising predominantly oxidative muscles, e.g. tibialias anterior 6. Here, we explored the possibility to lower the exercise intensity (force and frequency) and duration, to analyse the outcome with low-exercise protocols on the frequently studied – but predominantly glycolytic – plantarflexor muscle gastrocnemius medialis (GM), and to test what depletion is necessary to quantify τPCr.Methods
The metabolic and haemodynamic exercise response was simultaneously assessed in the calf, along GM, using multi-nuclear (1H/31P) data acquisition with high spatio-temporal resolution. Localised multi-voxel semi-LASER 31P MRS (VOIs = 25 cm3, TE = 29 ms) was interleaved with multi-slice 1H arterial spin labelling MRI (FAIR ASL, TE = 20 ms, 10 slices, d = 6 mm), yielding a multi-nuclear dataset every TR = 6 s. The protocol 7 was applied using a custom-built 31P/1H coil array in a 7 T MR Scanner (Siemens, Erlangen, Germany). Five young healthy subjects (4 male / 1 female) exercised twice in one scan session, each. Exercise force was of 15 – 28 % MVC, the prescribed standard exercise was 2 min, with two pushes per TR on a non-magnetic ergometer with adjustable force every 6 s; deviations from standard protocol see results.Results
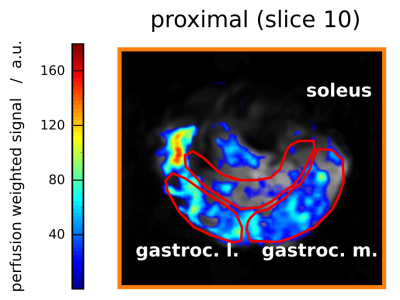
With this low-intensity exercise protocol, the 31P spectra localised to GM still showed PCr depletions of 46 ± 24 % (mean ± SD; range: 15 % – 83 %), very consistently at both voxel positions, see Fig. 1a. It was possible to reliably quantify τPCr with depletions as low as 19 %, see Fig. 2. Simultaneously acquired 1H MRI (Fig. 1b) showed increased perfusion in GM and gastrocnemius lateralis (GL), see Fig. 3, but this activity was smaller in all evaluated muscles (GM, GL and soleus) and across all slices than found in recently previously published data, that were obtained with the same MR sequence but with 3 min exercise at 30 % MVC 7. Different exercise durations were explored (3 min: pHee = 6.97, 1 min: pHee = 7.08), as well pushing once per TR for 3 min (6 % depletion: τPCr not quantifiable). There was however no correlation between exercise force or power and PCr depletion and, pHee or τPCr.Discussion
31P MRS results show the well-known pH decrease above ~60 % depletion (Fig. 2b) with comparable exercise protocols 3, associated with an increase of τPCr in acidified tissue (Fig. 2c). Interestingly, very short τPCr was found for small PCr depletions, particularly when pHee was alkaline due to PCr splitting. These short τPCrs were reliably quantified and similar in both voxels, placed adjacently in the same muscle (Fig. 1a, 3a). PCr depletion was matched by an increase in inorganic phosphate (Pi; data not shown) during exercise, and Pi decayed post exercise with similar time constants as PCr recovery, also for the shortest τPCrs. That PCr depletion and other parameters indicative for activation of GM were independent from prescribed force indicates that recruitment patterns are individually very different, especially at such small work levels. Apparently, exercise load can be distributed to other calf muscles than GM, although this muscle bears the main load with stronger exercise and a straight knee 4,8. Fast 31P spectroscopic imaging methods may resolve this issue if sufficient SNR is provided, while unlocalised MRS or large techniques with large VOIs may rather conceal differences in recruitment, potentially masking differences in pH and τPCr. To study the fast PCr recovery found with neutral to alkaline pHee, further studies employing shorter repetition times are desirable.Conclusion
Using low-force exercise protocols, small PCr depletions and neutral or even alkaline pH can be reached after 2 minutes. Even with depletions less than 20 %, the PCr recovery time remains reliably quantifiable and, given no acidification, was found to be as short as ~10 s or less, in several datasets.Acknowledgements
Funded by Austrian Science Fund (FWF) project I 1743-B13 to M.M.References
1. Kemp GJ, Ahmad RE, Nicolay K, Prompers JJ. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiol (Oxf). 2015;213(1):107-144.
2. Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab. 2012;37:88-99.
3. Fiedler GB, Schmid AI, Goluch S, Schewzow K, Laistler E, Niess F, Unger E, Wolzt M, Mirzahosseini A, Kemp GJ, Moser E, Meyerspeer M. Skeletal muscle ATP synthesis and cellular H+ handling measured by localized 31P-MRS during exercise and recovery. Sci Rep. 2016;6:32037.
4. Niess F, Fiedler GB, Schmid AI, Laistler E, Frass R, Wolzt M, Moser E, Meyerspeer M. Dynamic multivoxel-localized 31P MRS during plantar flexion exercise with variable knee angle. NMR Biomed. 2018;31(6):e3905.
5. Jeneson JAL, Bruggeman FJ. Robust homeostatic control of quadriceps pH during natural locomotor activity in man. The FASEB Journal. 2004;18:1010-1012.
6. Boss A, Heskamp L, Breukels V, Bains LJ, van Uden MJ, Heerschap A. Oxidative capacity varies along the length of healthy human tibialis anterior. J Physiol. 2018;596:1467-1483.
7. Niess F, Schmid AI, Bogner W, Wolzt M, Carlier M, Trattnig S, Moser E, Meyerspeer M. Interleaved 31P MRS / 1H ASL for analysis of metabolic and functional heterogeneity along human lower leg muscles at 7T. Magn Reson Med 2019; in press
8. Valkovič L, Chmelík M, Meyerspeer M, Gagoski B, Rodgers CT, Krššák M, Andronesi OC, Trattnig S, Bogner W. Dynamic 31P-MRSI using spiral spectroscopic imaging can map mitochondrial capacity in muscles of the human calf during plantar flexion exercise at 7 T. NMR Biomed. 2016 Dec;29(12):1825-1834
Figures
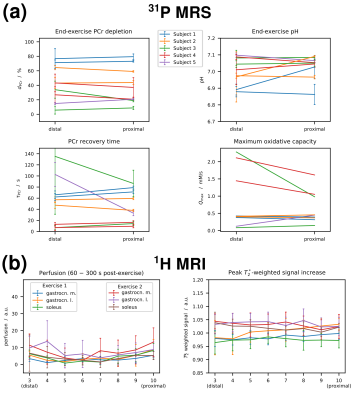

PCr recovery time (a) and end-exercise pH (b) plotted vs. end-exercise depletion, and PCr recovery time plotted versus end-exercise pH (c). Results from pairs of voxels selected in gastrocnemius medialis in a distal and a proximal position that were acquired during the same exercise bout are connected with a black line.
PCr recovery time
(τPCr)
was successfully fitted for PCr depletions above 18 %. Note that for very low depletions (and high end-exercise pH)
τPCr was found to be very short (on the order of 10 s), but was still reliably fitted (similar in both VOIs and consistent with Pi.)