0487
Cardiac 31P MR Spectroscopy With Interleaved 1H Image Navigation for Prospective Respiratory Motion Compensation – Initial Results1Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 2MR Center of Excellence, Medical University of Vienna, Vienna, Austria, 3Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria, 4Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria
Synopsis
Cardiac phosphorus (31P) magnetic resonance spectroscopy (MRS) offers unique insights into the metabolism of the human heart. To further improve cardiac 31P MRS acquisitions, the implementation of a proton (1H) magnetic resonance imaging (MRI) navigator into a 31P MRS pulse sequence using multinuclear interleaving is demonstrated. In this feasibility study we further apply a method to robustly detect the heart on low-resolution navigator images. Combined with multinuclear interleaving this facilitates time-efficient position updates of the 31P MRS voxel to prospectively correct for respiratory motion.
Introduction
In western countries, cardiovascular diseases are the leading cause of death. Phosphorus (31P) magnetic resonance spectroscopy (MRS) is the only tool to noninvasively detect and monitor pathological alterations of cardiac energy metabolism in vivo. 31P MRS has a very high specificity in predicting disease progression and patient survival, in e.g. heart failure1. The heart’s position and the poor filling factor due to its shape lead to low sensitivity and high susceptibility to motion because of its long measurement duration. To our knowledge, motion of the heart during the respiratory cycle is not yet compensated for during 31P MRS studies.Methods
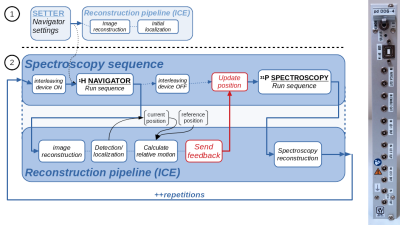
We modified the navigated spectroscopy framework as described previously2,3 to use a fast-low-angle-single-shot (FLASH) sequence (1.5 ms TE, 2.8 ms TR, 32 x 128 resolution, 200 x 200 mm2 FoV, 6 mm slice thickness, nominal flip angle 10°). Two perpendicular proton (1H) MRI slices in sagittal (PE: A->P) and coronal (PE: H->F) orientation were acquired as navigator to detect respiratory motion in all three spatial directions. Sequence parameters were optimized on a per-subject basis to yield maximal contrast at borders between the heart and neighboring tissue, primarily lung and liver, while maintaining total navigator acquisition times lower than 200 ms. An in-house developed algorithm4 to reproducibly localize the heart in these low-resolution images was implemented to the manufacturer's online reconstruction software. Detected in-plane positions were transformed to patient coordinates and combined using regularized singular-value decomposition. The reconstruction pipeline was modified to show detection results also in Syngo. For MRS acquisition, a stimulated echo acquisition mode (STEAM) pulse sequence was used. The navigator supplied real-time feedback for the voxel shift due to motion before each spectral acquisition (fig. 1)2. Multi-nuclear interleaving was achieved using the method described previously5, with the navigators being acquired as the second nucleus.3 healthy volunteers were measured on a 7 tesla (7T) MR scanner (Siemens Healthineers, Germany) equipped with a 1H/31P surface coil (31P-loop: 14 cm; Rapid Biomedical, Germany) using a STEAM pulse sequence (3000 ms TR, 3.7 ms TM, 7 ms TE, 40 x 20 x 45 voxel size, 128 averages, 90 degree flip angle, RF centered on PCr). Per-subject localized shimming of the whole heart was applied. Each repetition block of navigator and STEAM acquisition was triggered by the heartbeat using acoustic triggering6. Data were acquired by placing the MRS voxel in the interventricular septum as identified on CINE 2-chamber, 4-chamber and short-axis cardiac views (fig. 3). For each volunteer at least two measurements were performed, one with the navigator-feedback enabled and a second time without the position update. At the beginning of each measurement volunteers were asked to hold their breath in exhaled state for the first four scans to match the voxel to the planned position on the cardiac localisers.
Results
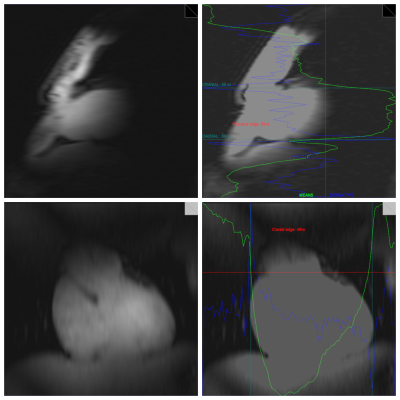
Localized 31P MR spectra with 1H navigator images and prospective update of the MRS voxel were successfully measured (fig. 3). For each MRS average, a navigator image and an annotated image showing the detection results were produced by the scanner’s image reconstruction unit (fig. 2). Depending on the volunteer’s body-composition, size and heart location, optimal parameters of the FLASH navigator sequence varied slightly resulting in a total navigator duration between 180 and 270 ms. The additional interval between navigator and MRS acquisition, which included reconstruction of the navigators, heart detection, composition of the displacement vector, feedback to the pulse sequence and update of the STEAM voxel position, took less than 120 ms. The acquired cardiac 31P MR spectra show good quality with PCr and γ-ATP peaks clearly detectable (fig. 3), other metabolites are displaced too strongly to be detectable.Discussion
We show proton-navigated 31P MRS data, as we believe, for the first time. The presented approach goes beyond respiratory gating or triggering for a time-efficient acquisition scheme. With these first measurements we prove the feasibility of multinuclear interleaved MRI-MRS acquisitions. The multi-dimensional heart detection and construction of a full 3D translational motion vector were implemented from scratch in the scanner’s ICE software and a modified framework for the online feedback handling2,3. Fast updates of MRS voxel positions using 1H MRI navigators allowed for scanning during end-systole which is optimal for SNR7. Next steps for further improvements include faster and additional navigator acquisitions, more robust and generic detection, a bigger coil8 as well as undertaking further careful tests and evaluations. We are confident that respiratory motion compensation combined with cardiac triggering will enhance the quality of cardiac 31P MR spectra, given that even with imperfect detection the quality and SNR at least matched non-navigated spectra.Conclusion
We show the feasibility of prospective 1H image-based respiratory motion compensation for cardiac triggered 31P MRS acquisitions in the human myocardium. The benefit of prospective motion compensation will be especially important for studying patients and during stress tests where irregular additional motion is to be expected.Acknowledgements
This project was supported by the Austrian Science Fund (FWF) project P28867-B30.References
1 Neubauer, S. (2007). The Failing Heart — An Engine Out of Fuel. New England Journal of Medicine, 356(11), 1140–1151. http://doi.org/10.1056/NEJMra063052
2 Hess, A. T., Dylan Tisdall, M., Andronesi, O. C., Meintjes, E. M., & van der Kouwe, A. J. W. (2011). Real-time motion and B0 corrected single voxel spectroscopy using volumetric navigators. Magnetic Resonance in Medicine, 66(2), 314–323. http://doi.org/10.1002/mrm.22805
3 Bogner, W., Hess, A. T., Gagoski, B., Tisdall, M. D., van der Kouwe, A. J. W., Trattnig, S., Rosen, B., Andronesi, O. C. (2014). Real-time motion- and B0-correction for LASER-localized spiral-accelerated 3D-MRSI of the brain at 3T. NeuroImage, 88, 22–31. http://doi.org/10.1016/j.neuroimage.2013.09.034
4 Körner, T., Wampl, S., Meyerspeer, M., Trattnig, S., Moser, E., Schmid, A. (2019, 3-5 Oct.). 3D heart localization for free breathing cardiac MR navigators in real time at 3T and 7T. In: ESMRMB 2019, 36th Annual Scientific Meeting, Rotterdam, NL, October 3–October 5: Abstracts, Saturday. (2019). Magnetic Resonance Materials in Physics, Biology and Medicine, 32(S1), 235–371. Abstract L06.10. https://doi.org/10.1007/s10334-019-00755-1
5 Meyerspeer, M., Magill, A. W., Kuehne, A., Gruetter, R., Moser, E., & Schmid, A. I. (2016). Simultaneous and interleaved acquisition of NMR signals from different nuclei with a clinical MRI scanner. Magnetic Resonance in Medicine, 76(5), spcone-spcone. https://doi.org/10.1002/mrm.26495
6 Frauenrath, T., Hezel, F., Renz, W., D’Orth, T. de G., Dieringer, M., von Knobelsdorff-Brenkenhoff, F., … Niendorf, T. (2010). Acoustic cardiac triggering: a practical solution for synchronization and gating of cardiovascular magnetic resonance at 7 Tesla. Journal of Cardiovascular Magnetic Resonance, 12(1), 67. https://doi.org/10.1186/1532-429X-12-67
7 Wampl, S., Körner, T., Meyerspeer, M., Moser, E., Trattnig, S., Schmid, A. (2019, 3-5 Oct.). Effect of trigger delay on human cardiac 31P MR spectra at 7T. In: ESMRMB 2019, 36th Annual Scientific Meeting, Rotterdam, NL, October 3–October 5: Abstracts, Saturday. (2019). Magnetic Resonance Materials in Physics, Biology and Medicine, 32(S1), 235–371. Abstract S23.07. https://doi.org/10.1007/s10334-019-00755-1
8 Goluch-Roat S, Vit M, Schmid AI, Laistler E. Simulation comparison of 28 different 31P arrays for cardiac MR spectroscopy at 7 T. In: Proceedings of the ISMRM 2019.
Figures
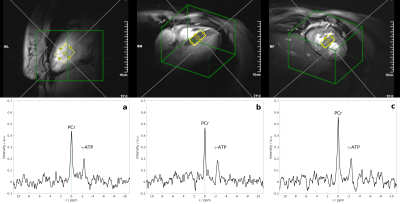
Top: Typical position of the MRS voxel (yellow) in the interventricular septum and the shim volume (green) seen on 2-chamber, 4-chamber and short axis views (left to right).
Bottom: Cardiac 31P MR spectra of a single volunteer for measurements performed without motion updates (a), with the default set of detection features (b) and with a set of manually adapted detection parameters (c). In all three spectra PCr and γ-ATP peaks can be clearly identified. STEAM, 36 ml voxel, 128 averages, 3 s TR, 3.7 ms TM, 7 ms TE.