0472
Mapping neuronal activity associated with finger tapping using direct measurement of 17O at 7 Tesla: proof-of-concept experiment1Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 2Radiology, German Cancer Research Center, Heidelberg, Germany, 3Faculty of Medicine, University of Heidelberg, Heidelberg, Germany, 4Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 5Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 6Institute of Medical Physics, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
Synopsis
Dynamic 17O-MRI enables direct quantification of the cerebral metabolic rate of oxygen (CMRO2) consumption. We investigated hemispherical dependence of the method in three healthy volunteers as well as its potential for mapping neuronal activity associated with finger tapping in one healthy volunteer. Our findings were consistent with previous results, demonstrating higher CMRO2 values in gray compared to white matter. Evaluation of left/right hemispheric CMRO2 values without sensomotoric stimulation demonstrated hemispherical independence of the technique. The finger-tapping experiment demonstrated increased 17O-signal in the stimulated sensorimotor cortex and adjacent brain tissue, indicating that dynamic 17O-MRI may permit visualization of physiological neuronal activity.
Purpose
Dynamic 17O-MRI enables metabolic imaging with reproducible quantification of the cerebral metabolic rate of oxygen (CMRO2) consumption in healthy volunteers 1 and patients with glioma 2. This imaging technique requires inhalation of enriched 17O2 gas. Data of three healthy volunteers were investigated with regard to hemispherical dependence of CMRO2 quantification and robustness of the method. Mapping of task-related neuronal activity was investigated in one healthy volunteer employing finger tapping and dynamic 17O-MRI.Methods
All experiments were performed on a 7-T whole-body MR system (Siemens, Erlangen, Germany). For the investigation of hemispherical dependence, three healthy volunteers (44±21 years, all men) were included in this study (n=3, 6 datasets). A home-built double-resonant 17O/1H head coil was used to acquire dynamic 17O data as well as basic proton images. A 24-channel 1H head coil (Nova Medical, Wilmington, Massachusetts) was used to obtain high spatial resolution anatomical data. Oxygen MRI data were acquired with a density-adapted radial sequence 3 with a Golden Angle distribution of projections 4 (nominal spatial resolution of (7.5mm)3, TR/TE=20ms/0.56ms, acquisition time t=40min). Administration of ca. 4.0L of 70%-enriched 17O2 gas enabled the determination of the cerebral metabolic rate of oxygen (CMRO2) consumption via three-phase inhalation experiment (baseline phase, 17O2 inhalation phase, decay phase) 1,5. The segmentation masks for gray matter (GM) and white matter (WM) were created automatically using the FSL-FAST algorithm tool 6 and were used for partial volume correction 7. All segmentation masks were subdivided into two compartments (left and right hemisphere) to investigate possible hemispherical dependence of CMRO2 values. The CMRO2 values for gray and white matter as well as their hemispherical dependence (left versus right hemisphere) were tested using Wilcoxon signed-rank tests with a level of significance of p<0.05. The experiment for mapping neuronal activity included dynamic 17O-MRI in one healthy volunteer (68 years, male). A finger-tapping paradigm was employed for stimulating the sensorimotor cortex. The chosen paradigm alternated blocks of resting and sequential right-hand finger-to-thumb tapping. A total of 40 images were acquired in 40 blocks of 60 seconds with the same experimental setup as for investigation of regional dependence.Results
Dynamic 17O-signals relative to the baseline show similar increases in the left and right brain hemispheres (LH, RH) for both gray matter and white matter (Fig. 1A). Increased CMRO2 in gray matter (2.38±0.15 μmol/g/min) was observed compared to white matter (0.63±0.05 μmol/g/min) (p=0.03) (Fig. 1B). Furthermore, the obtained CMRO2 values in gray matter and white matter are consistent with previous studies in healthy volunteers (gray matter: 1.42-3.57 μmol/g/min, white matter: 0.67-0.75 μmol/g/min) 1,5,8. The comparison between left and right hemispheric CMRO2 in gray and white matter did not show any significant differences (p=0.50 and p=0.25) (Fig. 1B). The neuronal activity experiment with the finger-tapping paradigm (right-hand tapping) employing dynamic 17O-MRI yielded higher 17O-signal increases in the left sensorimotor cortex and adjacent brain tissue compared to the contralateral brain region as well as the experiment without finger tapping (Fig. 2).Discussion
Dynamic 17O-MRI represents a non-invasive method to specifically quantify oxygen-dependent energy metabolism. Our findings for 17O-MRI of three healthy volunteers without sensomotoric stimulation showed that CMRO2 was higher in gray matter compared to white matter, without significant differences between the left and right hemisphere. This indicates hemispherical independence of the employed dynamic 17O-MRI technique. The high increase in 17O-signal in brain regions related to sensomotoric stimulation during finger tapping suggests that dynamic 17O-MRI may enable visualization of task-related neuronal activity in the healthy human brain. This finding is in agreement with a previous study in which mapping of neuronal activity was shown to be feasible using visual stimulation 9.Conclusion
Dynamic 17O-MRI demonstrates potential as a robust and non-invasive MRI technique that enables direct metabolic imaging in humans. The finger-tapping experiment in one healthy volunteer showed increased 17O-signal in the stimulated brain region. Therefore, dynamic 17O-MRI may permit visualization of physiological neuronal activity.Acknowledgements
The authors would like to thank NUKEM Isotopes GmbH for their supply of 17O2 gas and support of this project.References
1. Niesporek, SC, et al. Magn Reson Med 2018; 79: 2923–2934;
2. Niesporek SC, et al. In Proc. ISMRM 2018; #625;
3. Nagel, AM, et al. Magn Reson Med 2009; 62: 1565-1573;
4. Chan, RW, et al. Magn Reson Med 2009; 61(2): 354-63
5. Atkinson IC and Thulborn KR. Neuroimage 2010; 51: 723-33;
6. Zhang, Y, et al. IEEE Trans Med Imaging 2001; 20: 45-57;
7. Niesporek, SC, et al. Neuroimage 2015;112: 353-363;
8. Hoffmann SH, et al. MAGMA 2014; 27: 579-87;
9. Zhu, X, et al. In Proc. ISMRM 2006; #409;
Figures
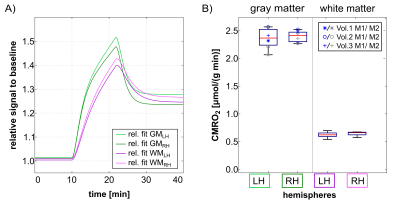
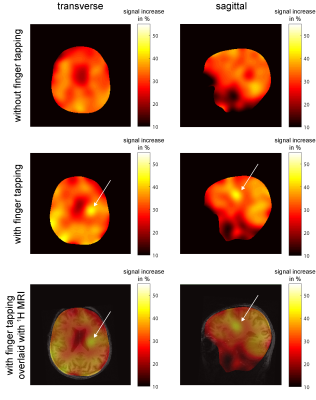
Fig. 2:
17O-signal increase between the beginning of the inhalation phase and the
end of the inhalation phase (in %). The map without stimulation by finger tapping shows uniform distribution of signal increase; for the experiment with stimulation there is a high signal
increase in brain regions related to sensomotoric stimulation. In the bottom
row, the map with finger tapping is overlaid with an anatomical MPRAGE image.