0468
Preserved high resolution brain MRI by data-driven DISORDER motion correction1Centre for the Developing Brain and Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain and Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences / MR Research Collaborations, King's College London / Siemens Healthcare Limited, London / Frimley, United Kingdom, 3Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Retrospective motion correction is applied for preserved image resolution on ultra-high field volumetric in-vivo brain MRI. Correction is based on the synergistic combination of appropriate view reorderings for increasing the sensitivity to motion and aligned reconstructions for deconvolving the effect of motion. Resolution loss introduced by motion is reverted without resorting to external motion tracking systems, navigators or training data. Contrast and sharpness improvements are shown on high resolution flow and susceptibility sensitive T1- and T2*-weighted spoiled gradient echo sequences acquired on cooperative volunteers.
Introduction
Sub-millimetric brain MRI is inherently compromised by unavoidable small subject motion, which may be exacerbated by long scan times1,2. Solutions based on optical tracking systems or navigators have been employed for prospective or retrospective rigid-body corrections at these scales. These generally show satisfactory performance but are respectively limited by the need of intrusive markers and constraints imposed by the imaging sequence3. The Distributed and Incoherent Sample Orders for Reconstruction Deblurring using Encoding Redundancy (DISORDER) method has been proposed for volumetric Cartesian brain imaging in the presence of motion, showing improved imaging reliability for main structural sequences in paediatric populations scanned without sedation at conventional imaging resolutions and standard accelerations4. Here, the application of this technique is extended to high resolution 7T data.Methods
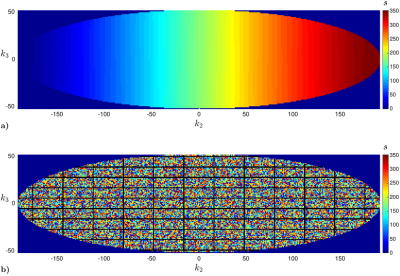
DISORDER sampling schemes (see Fig. 1) have been prototyped for spoiled gradient echo sequences on a 7T scanner (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany) and applied to volumetric brain imaging of consented healthy volunteers instructed to lay still. Human subject scanning was approved by the Institutional Research Ethics Commitee (HR-18/19-8700). Sequence 1: T1-weighted, Δ=0.45mm isotropic resolution, repetition time TR=9.2ms, echo time TE=3.9ms, flip angle α=17°, coronal field of view FOV 240×72×200mm3 (IS/AP/LR), scan duration TA=8min48s (6s for steady state, 2 repeats), DISORDER motion resolution δ=1.5s (random-checkered order / tile size U=32×11). Sequence 2: T2*-weighted, Δ=0.45mm isotropic, TR=23ms, TE=14ms, α=10°, coronal FOV 240×72×200mm3 (IS/AP/LR), TA=21min51s (6s for steady state), δ=3.7s (random-checkered / U=32×11). Sequence 3: T1-weighted, Δ=0.45mm isotropic, TR=24ms, TE=6.6ms, α=19°, axial FOV 220×180×48mm3 (AP/LR/IS), TA=12min38s (6s for steady state), δ=2.1s (random-checkered / U=32×11). Reconstructions with and without motion correction are compared. 32-channel coil sensitivities are estimated from the collected data by integrating a vortex-free unwrapped5 virtual body coil6 into ESPIRIT7. Stable and accurate motion estimates are promoted respectively by grouping together 8 consecutive k-space sweeps and applying a 0.5-order fractional finite difference weighting of k-space residuals8. Resolution loss from application of rigid motion operator in reconstruction is prevented by usage of sinc interpolation9,10. Motion estimation and reconstruction are alternated using Levenberg-Marquardt and conjugate gradient solvers4. Susceptibility-weighted contrast in Sequence 2 is obtained as described in11.Results
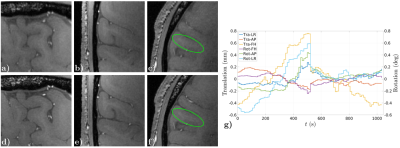
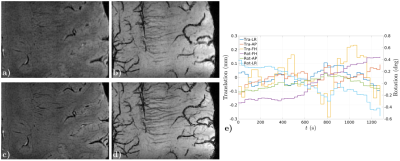
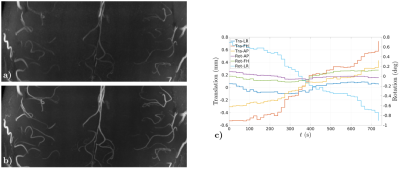
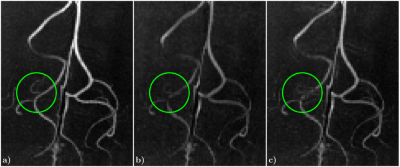
Figs. 2-4, corresponding respectively to scans using Sequences 1-3, show improved contrast and sharper delineation of fine-detailed structures when correcting for rigid motion. This can be noticed in better defined cortical structures (Fig. 2f versus 2c), improved white matter contrast (Fig. 3c versus 3a) and sharper and richer venous details (Figs. 3d versus 3b and 4b versus 4a). Reconstruction times were on the order of acquisition times. Motion traces suggest accurate estimates, with median excursions between subsequent motion states of 0.012° (Fig. 2g), 0.019° (Fig. 3e) and 0.016° (Fig. 4c). Moreover, in Fig. 2g estimates appear discontinuous at half the total scanning time, which is consistent with a small pause between the two scan repeats. Fig. 5 serves to corroborate these results by showing that quality degradation is observed for this particular level of motion and resolution scale irrespectively of the sampling modifications introduced by DISORDER.Discussion
We have shown improved in-vivo brain imaging resolution using data-driven aligned corrections at ultra-high field (UHF). Our estimates can track the rigid-body motion of the brain to a high spatial accuracy as inherited by the high resolution information collected within the sequence. However, limitations are expected for capturing quick and non-rigid motion due to insufficient gathered information and larger computational requirements when increasing the number of motion states and parameters. In addition, coarse scale motion at UHF may require corrections of high order effects12.Conclusion
We have demonstrated the feasibility of rigid motion corrected high resolution volumetric Cartesian brain imaging at 7T without resorting to navigators or specialized hardware. Future work will focus on extending our implementation to different sequences and on characterizing the limitations of our model at UHF.Acknowledgements
This work was supported by the ERC grant agreement no. [319456], the Wellcome/EPSRC Centre for Medical Engineering at King’s College London [WT 203148/Z/16/Z], the Wellcome Trust Collaboration in Science Award [201526/Z/16/Z], the Medical Research Council [MR/K006355/1], and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Budde J, Shajan G, Scheffler K, Pohmann R. Ultra-high resolution imaging of the human brain using acquisition-weighted imaging at 9.4 T. Neuroimage. 2014. 86:592‐8.
2. Stucht D, Danishad KA, Schulze P, Godenschweger F, Zaitsev M, Speck O. Highest resolution in vivo human brain MRI using prospective motion correction. PLOS One. 2015, 0133921:1-17.
3. Gretsch F, Mattern H, Gallichan D, Speck O. Fat navigators and Moiré phase tracking comparison for motion estimation and retrospective correction. Magn Reson Med. 2020. 83:83-93.
4. Cordero-Grande L, Ferrazzi G, Teixeira RPAG, O’Muircheartaigh J, Price AN, Hajnal JV. Motion corrected MRI with DISORDER: Distributed and Incoherent Sample Orders for Reconstruction Deblurring using Encoding Redundancy. arXiv. 2019. 1910.00540v1, 1-24.
5. Ghiglia DC, Romero LA, Robust two-dimensional weighted and unweighted phase unwrapping that uses fast transforms and iterative methods. J Opt Soc Am A. 1994. 11:107-17.
6. Buehrer M, Boesiger P, Kozerke S, Virtual body coil calibration for phased-array imaging. ISMRM. 2009. 17:760.
7. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRIT—An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014. 71:990-1001.
8. Loktyushin A, Nickisch H, Pohmann R, Schölkopf B. Blind multirigid retrospective motion correction of MR images. Magn Reson Med. 2015. 73:1457-68.
9. Unser M, Thévenaz P, Yaroslavsky L. Convolution-based interpolation for fast, high-quality rotation of images. IEEE Trans Image Proc. 1995. 4:1375-81.
10. Cordero-Grande L, Teixeira RPAG, Hughes EJ, Hutter J, Price AN, Hajnal JV. Sensitivity encoding for aligned multishot magnetic resonance reconstruction. IEEE Trans Comput Imag. 2016. 2:266-280.
11. Haacke EM, Tang J, Neelavalli J, Cheng YCN. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imag. 2010. 32:663-76.
12. Gretsch F, Marques JP, Gallichan D. Investigating the accuracy of FatNav-derived estimates of temporal B0 changes and their application to retrospective correction of high-resolution 3D GRE of the human brain at 7T. Magn Reson Med. 2018. 80:585-97.
Figures