0467
Scan-specific assessment of vNav motion artifact mitigation in the HCP Aging study using reverse motion correction1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 4Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
In studies that acquire a single prospectively-corrected scan it is unclear whether motion correction was beneficial when inspecting residual artifacts and the motion profiles. Here we used reverse motion correction to estimate images that would have resulted without vNav prospective motion correction (PMC). Matched motion tests were used to assess whether the reverse correction step was an accurate representation of images acquired during similar motion but without PMC. Using reverse motion correction on a subset of scans from the Human Connectome Project Aging study suggests that vNav PMC and selective reacquisition substantially improved image quality when there was motion.
Introduction
Performance of prospective motion correction (PMC) in neuroimaging is often unclear based on inspection of residual artifacts in a single “corrected” image together with the estimated head motion. During piloting or evaluation of PMC, studies may acquire another scan without PMC, but in general the profile of the motion will not be the same, so several pairs are required for statistical comparisons of image quality. During development of PMC sequences, experiments with repeated, similar motion will be performed to compare the images acquired with and without PMC. Neither of these approaches is specific to the exact motion that happened during the PMC scan and it is challenging to test the types of motion that occur in studies, or clinically, because they are difficult for volunteers to repeat.In this study, we used reverse motion correction1 to estimate the image that would have been acquired without PMC for the specific motion that happened during the scan. In matched motion comparisons with and without PMC, vNav with selective reacquisition2 has been shown to mitigate motion artifacts and thereby reduce the bias and variance in brain morphometry3. The specific value of the reacquisition component has been shown previously4,5. Here, we present a preliminary evaluation of vNav PMC in the Human Connectome Project Aging (HCP-A) study6,7 using the vNav PMC multi-echo MEMPRAGE k-space data.
Methods
Testing reverse motion correctionWe performed matched-motion experiments to assess the fidelity of the reverse motion correction procedure for the vNav MEMPRAGE protocol used in HCP-A. In experiments with 3 volunteers, small and discrete changes in head position were tested, as well as continuous head movement. Volunteers were scanned in accordance with the institutional review board guidelines. Two motion scans with and without vNav PMC, and a scan without intentional motion (PMC off), were acquired for each subject.
In volunteer experiments, the reverse motion corrected image was compared with the image acquired without motion correction. Also, it is expected that if retrospective (forward) motion correction of a scan without PMC8 does not resolve image artifacts for a particular profile of motion, then reversing PMC will also be inaccurate for that type of motion.
Data acquisition and reconstruction
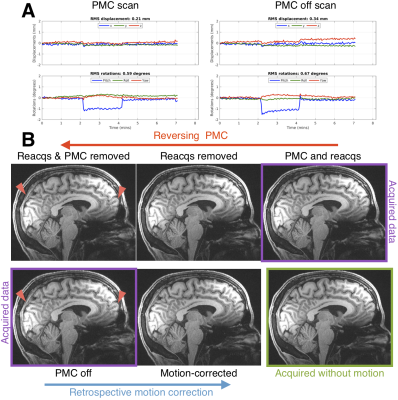
0.8mm isotropic resolution MEMPRAGE were acquired with TR/TI=2500/1000 ms, TE=1.8/3.6/5.4/7.2ms, and 744 Hz/px readout bandwidth6. A modified version of RetroMoCoBox8 (https://github.com/dgallichan/retroMoCoBox) was used to reconstruct images from k-space data acquired with R=2 GRAPPA acceleration. vNav motion information was extracted using a publicly available script (https://github.com/MRIMotionCorrection/parse_vNav_Motion). The following images were reconstructed from a vNav PMC scan:
- “PMC & reacqs”: IFFT of data acquired with PMC including reacquired data (up to 30 TRs) after check for lower RMS motion score2
- “Reacqs removed”: IFFT of data acquired with PMC excluding any reacquired data
- “Reacqs & PMC removed”: NUFFT based on vNav PMC of data excluding any reacquired data
The following images were reconstructed from scans without PMC that had vNav motion estimation enabled:
- “PMC off”: IFFT of data
- “Motion-corrected”: NUFFT based on vNav motion estimate for each TR (ky loop).
Evaluation of vNav PMC in HCP-A data
30 vNav PMC MEMPRAGE k-space datasets were collected at the Massachusetts General Hospital HCP-A site. The reverse motion correction procedure described above was performed.
For each comparison, images were registered to an unbiased template space9.
Results
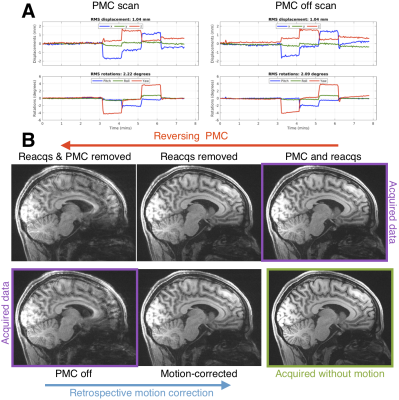
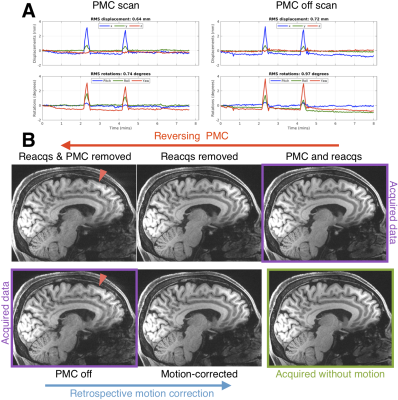
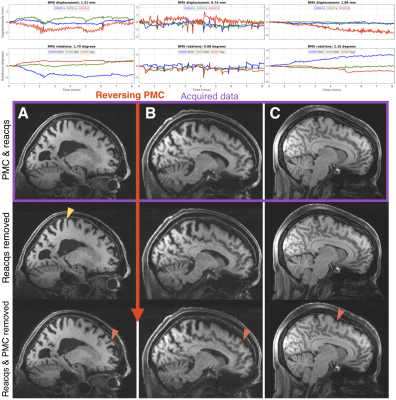
Figure 1 shows how two changes in head position can blur the “PMC off” image and suggests that reverse motion correction provides a good estimate of that image. Figure 2 shows that with larger motion reverse motion correction can provide an image with similar artifacts as the scan acquired without PMC. Note that the “Motion-corrected” image has residual artifacts so there may be inaccuracies in the reverse step. Figure 3 suggests that reverse motion correction can also estimate “PMC off” images when there is slow continuous motion.Figure 4 shows 3 examples of reverse motion correction in HCP-A data when there was substantial motion. Reacquisition improved image quality in Fig. 4A (note that this is the exact effect of not reacquiring data, i.e. it is not part of the reverse estimation). Fig. 4B suggests there would have been loss of grey-white contrast if vNav PMC had not been used and Fig. 4C suggests that vNav PMC reduced blurring.
Discussion
Reverse motion correction seems to provide a good approximation of images that would have been acquired without PMC (Figs. 1-3) although for larger or faster motion the approximation may break down and the image could contain “insufficient motion correction” artifacts that are not the “true” artifacts. The motion observed in the 3 HCP-A scans have similar profiles to what was tested so the reverse corrections could be an accurate approximation.Fig. 4B shows that there is some residual ringing but the reverse correction suggests that image quality was substantially improved. Also, inspection of the RMS motion summaries in the motion experiments and HCP subjects show that they are a poor indicator of image artifacts observed in experiments and with reverse correction.
Conclusions
Reverse motion correction is potentially powerful for assessment of PMC in neuroimaging studies and in clinical MRI. Further tests are required to assess the limits of the reverse correction step.Acknowledgements
We are grateful for access to data collected as part of the HCP Aging study and for the following funding sources: R01HD093578, R01HD085813, R01HD099846, R42CA183150, R00HD074649, U01AG052564, S10RR023401, S10RR019307, and S10RR023043.References
1. Zahneisen B, Keating B, Singh A, Herbst M, Ernst T. Reverse retrospective motion correction. Magn Reson Med 2016;75:2341–2349.
2. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med 2012;68:389–399.
3. Tisdall MD, Reuter M, Qureshi A, Buckner RL, Fischl B, van der Kouwe AJW. Prospective motion correction with volumetric navigators (vNavs) reduces the bias and variance in brain morphometry induced by subject motion. Neuroimage 2016;127:11–22.
4. Frost R, Hess AT, Okell TW, Chappell MA, Tisdall MD, van der Kouwe AJW, Jezzard P. Prospective motion correction and selective reacquisition using volumetric navigators for vessel-encoded arterial spin labeling dynamic angiography. Magn Reson Med 2016;76:1420–1430.
5. Sarlls JE, Lalonde F, Rettmann D, Shankaranarayanan A, Roopchansingh V, Talagala SL. Effectiveness of navigator-based prospective motion correction in MPRAGE data acquired at 3T. PLoS One 2018;13:e0199372.
6. Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, Coalson TS, Chappell MA, Dapretto M, Douaud G, Fischl B, Glasser MF, Greve DN, Hodge C, Jamison KW, Jbabdi S, Kandala S, Li X, Mair RW, Mangia S, Marcus D, Mascali D, Moeller S, Nichols TE, Robinson EC, Salat DH, Smith SM, Sotiropoulos SN, Terpstra M, Thomas KM, Tisdall MD, Ugurbil K, van der Kouwe A, Woods RP, Zollei L, Van Essen DC, Yacoub E. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage 2018;183:972–984.
7. Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, Burgess GC, Curtiss SW, Diaz-Santos M, Elam JS, Fischl B, Greve DN, Hagy HA, Harms MP, Hatch OM, Hedden T, Hodge C, Japardi KC, Kuhn TP, Ly TK, Smith SM, Somerville LH, Ugurbil K, van der Kouwe A, Van Essen D, Woods RP, Yacoub E. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage 2019;185:335–348.
8. Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magn Reson Med 2016;75:1030–1039.
9. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012;61:1402–1418.
Figures