0466
Comparison of Prospective and Retrospective Motion Correction for 3D Structural Brain MRI1DTU Compute, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Department of Clinical Physiology, Nuclear Medicine & PET, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4TracInnovations, Ballerup, Denmark, 5Department of Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
This work compares prospective and retrospective motion correction based on their capabilities to remove motion artifacts from 3D-encoded MPRAGE scans. Motion artifacts in clinical and research brain MRI are a major concern and the outcome of this problem includes repeated scans and the need for patient sedation or anesthesia, causing increased study time and cost. The prospective and retrospective correction approaches substantially improve the image quality of in-vivo scans for similar motion patterns. Prospective motion correction resulted in higher image quality than retrospective correction for larger discrete movements, and for periodic motion.
Introduction
Head motion is an ongoing problem in MRI brain imaging, causing reduced diagnostic image quality or biased research results1,2. Prospective Motion Correction (MoCo)3-5 and retrospective MoCo of k-space6 have shown promising results for improving image quality. Due to the different correction strategies of these two methods, there are both technical and workflow-related advantages and disadvantages7. Prospective MoCo in principle updates the image encoding in real-time to resolve k-space under-sampling that Retrospective MoCo suffers from, however, prospective MoCo requires pulse sequence modification and the uncorrected images are not available for comparison. Retrospective MoCo preserves the original images and if motion is measured with an external tracking system, it does not require customized sequences. In this work, we compared prospective and retrospective MoCo with matched-motion experiments. In addition, we also used simulations to assess retrospective correction.Methods
Rigid head motion was estimated using the Tracoline TCL3.01 markerless tracking system (TracInnovations, Ballerup, Denmark)8,9. Motion tracking and prospective correction were implemented on a 3T Prisma scanner (Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil. During the scan, 30 surface scans of the subject’s face were recorded per second. The tracking system estimates motion by finding the rigid transformation that aligns the current surface back to an initial reference surface. The motion estimates were geometrically aligned to the scanner by matching a reference surface to the surface of an MRI calibration scan.The k-space and motion data were saved from two scan sessions of five MPRAGE scans of healthy volunteers, who were scanned in accordance with Institutional Review Board guidelines. In both scan sessions, the two volunteers were instructed to move in a repeatable pattern. In session one, the pattern was discrete motion (Fig 2A) and in session two, it was periodic motion (Fig 3A). Within a session, matched comparisons of medium and larger amplitude movements were performed. In the last scan, the volunteers were instructed to remain motionless. The MPRAGE scans have the following protocol FOV=256x256mm2, matrix=256x256, 176x1mm sagittal slices, in-plane GRAPPA R=2, TR=2500ms, TE=3.3ms, TI = 1070ms, bandwidth=240Hz/px, echo spacing=8ms, and turbo factor=176.
Prospective MoCo was applied by modifying an MPRAGE sequence to adjust the imaging Field of View (FoV) according to motion estimation received from the tracker. The MoCo sequence adjusts its FoV before the start of each echo-train, and every six readouts thereafter9. The prospective MoCo sequence was reconstructed with the same reconstruction algorithm as used for the retrospective MoCo, to avoid dissimilarities due to scanner versus offline reconstruction methods.
Retrospective MoCo was performed using the freely available retroMoCoBox software package10. Each k-space line was aligned in time to the nearest available motion estimate before translations were corrected by adding additional phase ramps to each line. The non-uniform fast Fourier transformation11 was used to correct for rotations12.
Synthetic motion corrupted k-space data were simulated to further investigate the performance of the retrospective MoCo when the exact motion is known. Using code adapted from10, multi-channel k-space data from the motionless MPRAGE scan were used to generate k-space data during the presence of similar motion as that performed by the volunteers in the two sessions. The amplitude of the motion was varied. The generated k-space data were then reconstructed with and without retrospective MoCo. This simulation is sensitive to k-space under-sampling, but receiver coil sensitivity effects were not simulated.
Image quality was quantified by computing the Root Mean Square Error (RMSE) using the motionless MPRAGE scans as ground truth. A rigid registration (to motionless MPRAGE) and image normalization (zero mean and standard deviation one) were performed on every image prior to computing RMSE.
Results
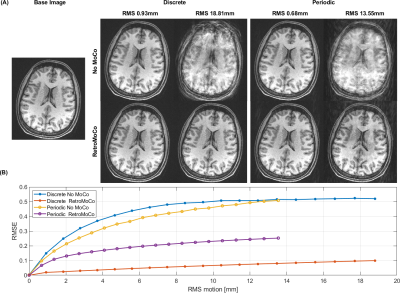
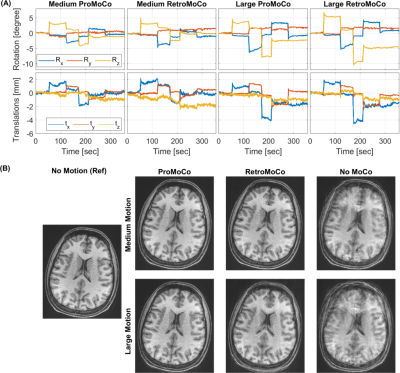
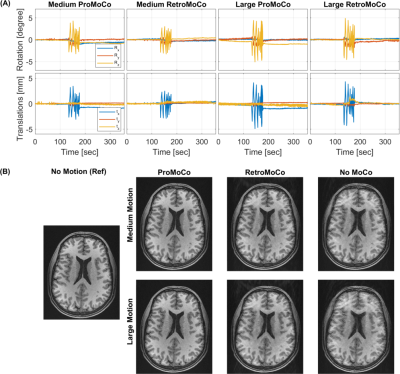
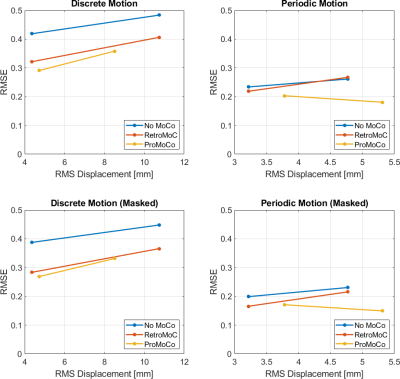
The motion simulations in Fig 1 show that retrospective MoCo removes the artifacts in the discrete motion case, however, in the case of periodic motion, retrospective MoCo was not able to remove all artifacts. The motion estimates in Fig 2 and 3 show that the motion was similar for each in vivo comparison, although there was a difference in the large discrete motion comparison. Fig 2B and 3B show that both prospective and retrospective MoCo provide improved image quality. However, in the case of periodic and large discrete motion, the retrospectively corrected images have some residual motion artifacts. Fig 4 shows that prospective and Retrospective MoCo reduced RMSE for both motion patterns when the backgrounds of the images were removed.Discussion
Prospective and retrospective MoCo both improved image quality during the discrete and periodic motions tested here. In these preliminary comparisons, the prospective MoCo images had less artifacts than the retrospective MoCo images. The subject was trained to repeat motion as similarly as possible, but for example, in the large discrete motion comparison, the motion was larger in the scan used for retrospective correction. Further, in vivo tests are required to definitively assess the performance of retrospective and prospective correction. The simulations suggest that periodic motion is more challenging to correct retrospectively. This was confirmed in the experiments, where the motion was well matched between the prospective and retrospective corrections.Conclusion
The tested prospective and retrospective MoCo approaches were able to reduce motion artifacts of 3D structural MPRAGE images. Prospective MoCo resulted in better image quality in repeated in vivo motion experiments.Acknowledgements
We are grateful for the following NIH funding: R01HD093578, R01HD085813, R01HD099846, R42CA183150.References
1. Andre JB, Bresnahan BW, Mossa-Basha M, et al.: Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol 2015; 12:689–695.
2. Tisdall MD,
Reuter M, Qureshi A, Buckner RL, Fischl B, van der Kouwe AJW: Prospective
motion correction with volumetric navigators (vNavs) reduces the bias and
variance in brain morphometry induced by subject motion. Neuroimage
2016; 127:11–22.
3. Thesen S, Heid O, Mueller E, Schad LR: Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med 2000; 44:457–465.
4. Zaitsev M, Dold C, Sakas G, Hennig J, Speck O: Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage 2006; 31:1038–1050.
5. van der Kouwe AJW, Benner T, Dale AM: Real-time rigid body motion correction and shimming using cloverleaf navigators. Magn Reson Med 2006; 56:1019–1032.
6. Gallichan D, Marques JP, Gruetter R: Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magn Reson Med 2016; 75:1030–1039.
7. Maclaren J, Herbst M, Speck O, Zaitsev M: Prospective Motion Correction in Brain Imaging: A Review. Magn Reson Med 2013; 69:621–636.
8. Slipsager JM, Ellegaard AH, Glimberg SL, et al.: Markerless motion tracking and correction for PET, MRI, and simultaneous PET/MRI. PLoS One 2019; 14:1–17.
9. Frost R, Wighton P, Karahanoğlu FI, et al.: Markerless high-frequency prospective motion correction for neuroanatomical MRI. Magn Reson Med 2019; 82:126–144.
10. RetroMoCoBox Toolbox [https://github.com/dgallichan/retroMoCoBox]
11. Implementation of Non Uniform Fast Fourier Transformation [https://github.com/andyschwarzl/gpuNUFFT]
12. Slipsager JM, Glimberg SL, Gallichan D, Højgaard L, Paulsen RR, Olesen OV: Clinical Implementation of Fast Markerless Motion Correction in K-Space of Structural 3D MR-Images of the Brain. Montreal: ISMRM; 2019.
Figures