0463
Scout Acquisition enables rapid Motion Estimation (SAME) for retrospective motion mitigation.1Department of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 2Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Navigation-free retrospective motion-correction typically requires estimating hundreds of coupled temporal motion parameters by solving a large non-linear inverse problem. This can be extremely demanding computationally, which has impeded implementation/adoption in clinical settings. We propose a technique that utilizes a single rapid scout scan (Tadd=3sec) to drastically reduce the computation cost of this motion-estimation and create a pathway for clinical acceptance. We optimized this scout along with the sequence acquisition reordering in a 3D Turbo-Spin-Echo acquisition. Our approach was evaluated in-vivo with up to R=6-fold acceleration and robust motion-mitigation was achieved using a scout with differing contrast to the imaging sequence.
Introduction
Navigator-free retrospective motion correction techniques [1]–[5] often perform image reconstruction by solving$$\min_\vec{\theta}\min_x\|\sum_iM_iFCT_{\theta_i}R_{\theta_i}x-k_i\|_2\qquad\quad[1]$$ where $$$k_i$$$ denotes the multi-channel k-space data of shot $$$i$$$, $$$x$$$ the image, $$$T_{\theta_i}R_{\theta_i}$$$ the shot-dependent translation and rotation motions, $$$C$$$ the coil sensitivity, $$$F$$$ the Fourier transformation and $$$M_i$$$ the undersampling mask. All shots are coupled in this inverse problem and the non-convex estimation of several hundreds of temporal motion parameters is computationally demanding.
Substantial speed-up was demonstrated in NAMER [6], which can solve for the motion parameters in each shot separately using a good initial image estimate $$$\hat{x}$$$, obtained via e.g. Machine Learning.
$$\min_{\vec{\theta}_i}\|M_iFCT_{\theta_i}R_{\theta_i}\hat{x}-k_i\|_2\qquad\qquad\quad[2]$$ However, for this method to work robustly, large amounts of training data (motion-corrupted & motion-free) are needed to enable generalizability to arbitrary motion patterns. Moreover, repeated updates of the motion parameters $$$\vec{\theta}$$$ are typically required to refine the Machine Learning generated image $$$\hat{x}$$$ and arrive at the correct motion estimate.
In this work, we propose an alternative strategy, termed SAME, which does not rely on pre-trained Machine Learning networks. Inspired by traditional navigator-based motion correction techniques [7]–[9], SAME estimates the motion parameters quickly using a single low-resolution scout scan $$$\hat{x}$$$. We optimized this scan along with the sequence acquisition reordering of our target 3D Turbo-Spin-Echo acquisition, to achieve accurate motion estimation while minimizing added scan time (Tadd=3sec). We then demonstrate motion-mitigation capability of this approach in accelerated 3D-TSE scans with up to R=6-fold acceleration and achieve motion-robust rapid imaging via Wave-encoding.
Methods
Sequence ordering: We optimized the encoding reordering of our prototype T2w SPACE sequence to improve the robustness of its motion estimation. To test different reorderings, motion-free data with linear and two types of radial reorderings (with VDS-Poisson) were acquired at 1 mm isotropic resolution and R=4 acceleration using a 3T scanner (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany) and 64-channel head coil. After instructed subject motion, a separate motion-free scout scan $$$\hat{x}$$$ was acquired (same protocol) and used to estimate inter-scan motion by minimizing Eq. 2 (‘fminunc’ in MATLAB). Motion estimation with respect to the scout scan was performed for each shot (TR) within the T2w SPACE acquisition which should lead to identical motion-estimates across all TRs.Scout scan: We optimized the scout acquisition to minimize added scan time. Specifically, T2w scouts with&without dummy shots were acquired at 1x4x4 mm3 resolution and R=9-fold acceleration, achieved with Wave-CAIPI [10]. We then estimated inter-scan motion parameters between the scout and imaging sequence and analyzed how image contrast differences between these scans (due to differences in acquisition reordering and the absence of dummy-shots) influence motion estimation accuracy.
Inter-shot motion experiments: Based on the findings from above, we utilized radial reordering for all motion corrupted in-vivo acquisitions and added a rapid R=9 Wave-scout prior to each imaging sequence (Tadd=3sec). For T2w SPACE, acquisitions were performed at R=4 and R=6-fold acceleration (VDS-Poisson, with&without Wave). For motion-corrupted FLAIR-SPACE (R=6, Wave) we also assessed the motion estimation provided by either a T2w or FLAIR-weighted scout scan.
Results
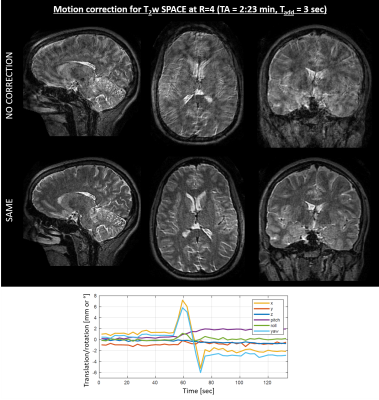
Linear reordering does not provide enough k-space overlap between the low-resolution scout scan (yellow) and the shot data (white), resulting in poor motion parameter estimates (Fig. 1a). Better results were obtained for radial reordering (one spoke), however best agreement with the ground truth was observed for two radial spokes. Removing dummy shots in the acquisition of our low-resolution scout caused substantial contrast differences (Fig. 1b). This, however, did not seem to affect the motion estimation.Figure 2 shows the inter-shot motion results for T2w SPACE at R=4-fold acceleration. The SENSE reconstruction demonstrates substantial motion artifacts and loss of contrast/resolution, which was largely mitigated using SAME. The fully-separable motion-estimation took TØ=91sec per shot using unoptimized MATLAB-code which is readily parallelizable.
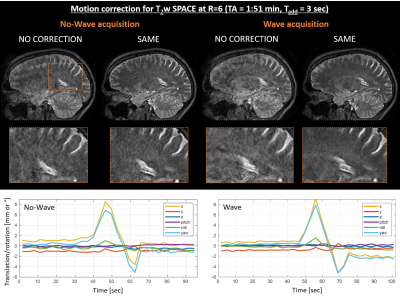
Figure 3 demonstrates the benefit of integrating Wave-encoding into the imaging sequence. At R=6, Wave-encoding better suppressed noise amplification (zoom-in) and achieved good image quality with substantial reduction of motion artifacts. The motion-estimation on Wave data took TØ=2:53min per shot (MATLAB), again readily parallelizable.
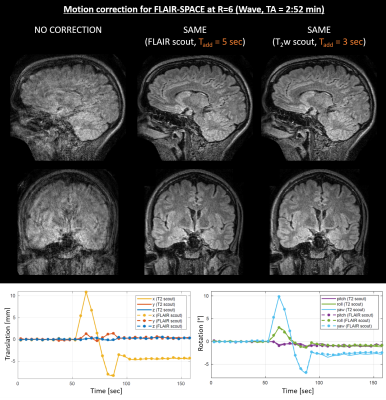
In Fig. 4, we tested our approach for FLAIR-SPACE (R=6, Wave). As evident by the SAME reconstructions and motion trajectory plots, both T2w and FLAIR scout acquisitions enabled accurate motion estimation for this FLAIR-SPACE dataset.
Discussion
In this work, we proposed a novel motion-correction technique for 3D acquisitions where the usage of a rapid scout dramatically reduced the computational footprint of the motion parameter estimation. We evaluated this technique in-vivo on T2w SPACE data and demonstrated robust motion estimation and correction without requiring any updated reconstruction of the imaging volume during motion-estimation [5],[6]. This enables parallelization across all shots pointing to good potential for online reconstruction with clinically acceptable reconstruction time (e.g., T=91sec*Nshots/Nthreads in MATLAB). Moreover, we showed the benefits of Wave-encoding in highly-accelerated scout and imaging acquisitions. We also tested our approach for FLAIR-SPACE where robustness to contrast variations between the scout and imaging sequence was observed. This may eliminate the need for scout re-acquisition across the clinical exam, where several imaging contrasts are acquired. Future work will examine potential improvements from using more incoherent sampling orders [11] and adoption into other 3D Wave sequences (e.g., MPRAGE [12], GRE [10]) which could enable a rapid motion-robust 3D brain exam [13].Acknowledgements
This work was supported in part by NIH research grants: R01EB020613, R01EB019437, R01 MH116173, P41EB015896, U01EB025162 and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043.References
[1] M. W. Haskell, S. F. Cauley, and L. L. Wald, “TArgeted Motion Estimation and Reduction (TAMER): Data consistency based motion mitigation for mri using a reduced model joint optimization,” IEEE Trans. Med. Imaging, vol. 37, no. 5, pp. 1253–1265, 2018.
[2] A. Loktyushin, H. Nickisch, R. Pohmann, and B. Schölkopf, “Blind multirigid retrospective motion correction of MR images,” Magn. Reson. Med., 2015.
[3] L. Cordero-Grande, R. P. A. G. Teixeira, E. J. Hughes, J. Hutter, A. N. Price, and J. V. Hajnal, “Sensitivity Encoding for Aligned Multishot Magnetic Resonance Reconstruction,” IEEE Trans. Comput. Imaging, 2016.
[4] D. Atkinson, D. L. G. Hill, P. N. R. Stoyle, P. E. Summers, and S. F. Keevil, “Automatic correction of motion artifacts in magnetic resonance images using an entropy focus criterion,” IEEE Trans. Med. Imaging, 1997.
[5] L. Cordero-Grande, E. J. Hughes, J. Hutter, A. N. Price, and J. V. Hajnal, “Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: Application to neonatal brain imaging,” Magn. Reson. Med., 2018.
[6] M. W. Haskell et al., “Network Accelerated Motion Estimation and Reduction (NAMER): Convolutional neural network guided retrospective motion correction using a separable motion model,” Magn. Reson. Med., vol. 82, no. 4, pp. 1452–1461, 2019.
[7] D. Gallichan, J. P. Marques, and R. Gruetter, “Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T,” Magn. Reson. Med., 2016.
[8] R. Bammer, M. Aksoy, and C. Liu, “Augmented generalized SENSE reconstruction to correct for rigid body motion,” Magn. Reson. Med., 2007.
[9] M. D. Tisdall, A. T. Hess, M. Reuter, E. M. Meintjes, B. Fischl, and A. J. W. Van Der Kouwe, “Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI,” Magnetic Resonance in Medicine. 2012.
[10] B. Bilgic et al., “Wave-CAIPI for highly accelerated 3D imaging,” Magn. Reson. Med., vol. 73, no. 6, pp. 2152–2162, 2015.
[11] L. Cordero-Grande, G. Ferrazzi, R. P. A. G. Teixeira, J. O’Muircheartaigh, A. N. Price, and J. V. Hajnal, “Motion corrected MRI with DISORDER: Distributed and Incoherent Sample Orders for Reconstruction Deblurring using Encoding Redundancy,” Oct. 2019.
[12] D. Polak et al., “Wave-CAIPI for highly accelerated MP-RAGE imaging,” Magn. Reson. Med., vol. 79, no. 1, pp. 401–406, 2018.
[13] D. Polak et al., “Highly-accelerated volumetric brain protocol using optimized wave-CAIPI encoding,” in Proc. Intl. Soc. Mag. Res. Med., 2018, p. 0937.
Figures