0461
Motion monitoring using MR-compatible ultrasound-based sensors1Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
The purpose of this work is to develop MR-compatible sensors that can attach to the patient’s skin, to monitor breathing in a comprehensive manner. In contrast, alternatives such as MRI navigator echoes and optical tracking are typically rigidly fixed to walls or floors and as such cannot accompany a given patient through serial diagnostic and/or therapeutic procedures. We show here that these sensors capture breathing motion in much of its complexity, as validated against MRI and optical tracking data. These sensors could be used to help combine information from different modalities in a manner that takes internal motion into account.
Introduction
Medical procedures can be difficult to perform on anatomy that is constantly moving. Respiration displaces internal organs by up to several centimeters1,2, and patients often have limited ability to hold their breath3-4. Current devices to monitor respiration, such as optical tracking5-7, may detect changes on the outline of the body rather than internal motion itself. MRI-based motion monitoring8,9 can lengthen scan times, and is feasible only while the patient happens to lie within an MRI scanner. Here we show that small ultrasound-based sensors referred to as ‘organ configuration motion’ (OCM) sensors10 can characterize internal motion directly, inside and outside the MRI suite. They are relatively inexpensive to build, unlike cameras they do not require an open line of sight, and because they are attached to the skin they can follow patients through successive procedures. The present work aimed to validate the respiration waveforms these sensors provide against well-established methods such as MRI and optical tracking.Methods
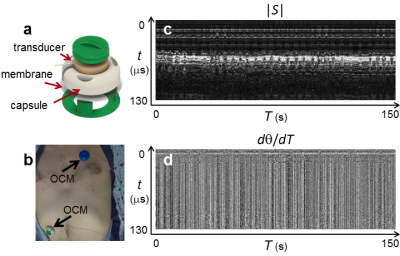
Thirteen volunteers were recruited. Following informed consent, an OCM sensor (Fig. 1a) accompanied subjects A1 through A5 inside the bore of a 3T MRI scanner. Subjects B1-B8 lied on the couch of a CT scanner instead (no CT scanning performed), in a Radiation Oncology simulation room equipped with a ceiling-mounted optical tracker.OCM signals are two-dimensional (Fig. 1c,d): an axis t captures the signal evolution after sensor firing, and an axis T captures changes from one sensor firing to the next (∆t=10-8s and ∆T=10-2s). Processing was causal, i.e., treating time point T0 involved only data from time points T≤T0. OCM signals were converted into velocity measurements, as in Doppler imaging11:
(1) V = (λ ⁄ 2π)×(∆θ ⁄ ∆T),
where θ(t,T) is the phase of the OCM signal (e.g., Fig. 1d). A one-dimensional trace was created by applying a median operator along t:
(2) vr (T) = median(V(tr,T)),
where tr represents a selection of t values: either the whole range corresponding to 10cm of depth, or a third of that range (0 to 3.3cm, 3.3 to 6.7cm, or 6.7 to 10cm). A breathing waveform was obtained through integration:
(3) z(T) = ∫ v(T ')dT ' (integrated from 0 to T).
Time integrals are susceptible to drifts as any problem at a given T0 propagates to all T>T0. De-trending of z(T) was performed based on OCM magnitude signals, |S(t,T)|. For each time point Ti,1, up to three past points Ti,j < Ti,1 (where j>1) were identified that minimized the function f below:
(4) f = ∑t|S(t,Ti,1)-S(t,Ti,j)|.
When considering a given time point T0 (i.e., ‘now’), only recent pairs such that (T0-Ti,1)< 5s and (T0-Ti,j)< 8s were considered. For such qualifying pairs a slope was calculated:
(5) mi = (z(Ti,1)-z(Ti,j)) ⁄ (Ti,1-Ti,j).
Assuming that time points with similar magnitude should correspond to similar breathing-related displacements, a non-zero slope in Eq. 5 would be caused by undesired drifts. Information about mi at all T<T0 time points was used to calculate a corrected version of the waveform z(T) from Eq. 3, zc(T).
The same algorithm and settings were used for all subjects. MRI, optical tracking and OCM signals measured different physical aspects of the breathing motion: displacements of the right hemi-diaphragm, motion of the surface of the torso, and relative motion of internal tissues with respect to skin locations, respectively. One global linear fit was performed to bring MRI and optical tracking breathing waveforms to the same scale, in mm, as corresponding OCM-derived ones.
Results
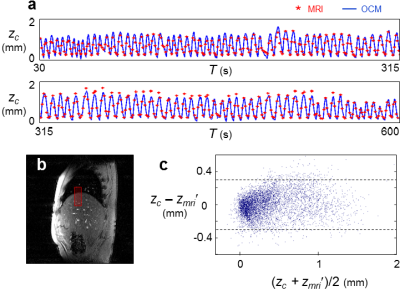
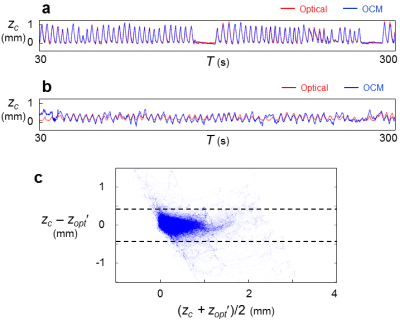
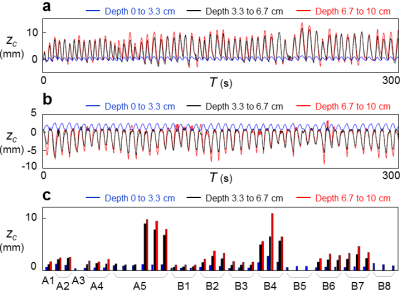
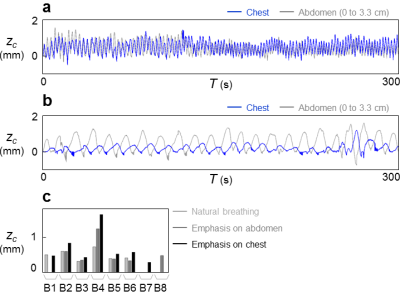
Results for subjects imaged by MRI+OCM are shown in Fig. 2. MRI-based breathing traces were obtained by placing an ROI over the right hemi-diaphragm in the sagittal plane (Fig. 2b). A typical dataset (median error) is shown in Fig. 2a. OCM-based result, zc, and corresponding MRI-based results, zmri, are compared in Fig. 2c for all subjects (A1 through A5). The 95% limits of agreement were 0.59 mm apart and the mean absolute error was 0.11 mm. Results for optical tracking+OCM are shown in Fig. 3. A best case and a typical (median error) case are presented in Fig. 3a and 3b, respectively. The comparison between OCM and corresponding optical tracking results, zopt, is shown in Fig. 3c for all subjects (B1 through B8). The 95% limits of agreement were 0.85 mm apart and the mean absolute error was 0.13 mm. Abdominal motion at three separate depths is plotted in Fig. 4a and 4b for two different subjects (A5 and B4). The amplitude of abdominal motion detected at all depths is shown in Fig. 4c, whenever available, for all subjects. Chest motion is shown in Fig. 5 for two different subjects (B2 and B8) and all available chest-related information is summarized in Fig. 5c.Discussion and Conclusion
OCM-derived results essentially included the information from MRI and optical tracking (Figs. 2 and 3) yet captured further information (Figs. 4 and 5). Unlike MRI or optical tracking, OCM sensors can accompany a given patient through diagnostic and therapeutic procedures separated in time and space. More specifically, because they are fixed to the skin rather than to floors or walls, the sensors can potentially help connect/fuse data from matched breathing state even if acquired hours or days apart, in different scanners.Acknowledgements
This work was supported in part by NIH grants P41EB015898 and R03EB025546.References
1. Wade OL. Movements of the thoracic cage and diaphragm in respiration. J Physiol 1953;124:193-212.
2. Suramo I, Paivansalo M, Myllyla V. Cranio-caudal movements of the liver, pancreas and kidneys in respiration. Acta Radiol Diagn (Stockh) 1984;25(2):129-131.
3. Gay SB, Sistrom CL, Holder CA, Suratt PM. Breath-holding capability of adults. Implications for spiral computed tomography, fast-acquisition magnetic resonance imaging, and angiography. Invest Radiol 1994;29(9):848-851.
4. Holland AE, Goldfarb JW, Edelman RR. Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology 1998;209(2):483-489.
5. Wagman R, Yorke E, Ford E, Giraud P, Mageras G, Minsky B, Rosenzweig K. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys 2003;55(3):659-668.
6. Spangler-Bickell MG, Khalighi MM, Hoo C, DiGiacomo PS, Maclaren J, Aksoy M, Rettmann D, Bammer R, Zaharchuk G, Zeineh M, Jansen F. Rigid Motion Correction for Brain PET/MR Imaging using Optical Tracking. IEEE Trans Radiat Plasma Med Sci 2019;3(4):498-503.
7. Zaitsev M, Dold C, Sakas G, Hennig J, Speck O. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage 2006;31(3):1038-1050.
8. Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989;173(1):255-263.
9. Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med 1999;42:963-969.
10. Preiswerk F, Toews M, Cheng CC, Chiou JG, Mei CS, Schaefer LF, Hoge WS, Schwartz BM, Panych LP, Madore B. Hybrid MRI-Ultrasound acquisitions, and scannerless real-time imaging. Magn Reson Med 2017;78(3):897-908.
11. Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. Doppler ultrasound. In: Jerrold T. Bushberg JAS, Edwin M. Leidholdt and John M. Boone, editor. The essential physics of medical imaging. Philadelphia, PA, USA: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2012. p 542-554.
Figures