0460
Non-rigid Motion Correction for Fetal Body MRI1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Motion correction for fetal body MRI is particularly challenging due to non-rigid deformations of organs caused by bending and stretching. Rigid slice-to-volume registration (SVR) methods are efficient for 3D fetal brain reconstruction. However, for full body reconstruction, misregistration errors caused by deformable motion lead to degradation of features. We propose a novel deformable SVR (DSVR) method based on hierarchical deformable registration for reconstruction of 3D fetal trunk from multiple motion corrupted stacks. The method is quantitatively evaluated by comparison to the state-of-the-art methods on 20 iFIND fetal MRI datasets. Furthermore, DSVR reconstruction quality is assessed on 100 fetal MRI cases.
Introduction
Motion correction for fetal body MRI poses a particular challenge due to local non-rigid deformations of organs caused by bending and stretching [1]. Classical rigid slice-to-volume registration (SVR) [2,3] methods provide an efficient solution for 3D super-resolution (SR) reconstruction of fetal brain since it undergoes only rigid transformations. Recently, SVR was also employed for reconstruction of 3D fetal thorax under the rigid motion assumption [4] and piece-wice rigid patch-to-volume registration (PVR) [5] method was proposed for large FoV motion correction. However, for full body reconstruction, misregistration errors caused by deformable motion lead to degradation of features and severe blurring in the output volumes. In this work, we propose a deformable SVR (DSVR) to reconstruct a high-resolution 3D volume of fetal body from motion corrupted stacks of slices of multiple orientations, which extends our earlier method for respiratory motion correction in single stack abdominal MRI [6].Method
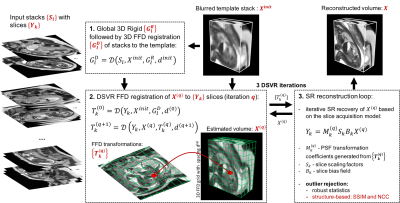
To account for deformation of the fetal body during acquisition we integrate free form deformation (FFD) [8] registration into the SVR pipeline. Correction of both in- and out-of-plane motion is ensured by registration of the volume to the slices rather than the slices to the volume. The under constrained nature of the problem due to the unknown fetal trunk shape is addressed by a hierarchical non-rigid SVR SR scheme with structure-based outlier rejection. The main components of DSVR pipeline are shown in Fig.1. The input includes motion-corrupted stacks {$$$S_l$$$}$$$_{l=1,..,L}$$$ with {$$$Y_{k}$$$}$$$_{k=1,..,K}$$$ slices and a mask covering the fetal trunk ROI. The least motion corrupted stack is selected as a template: $$$X^{init}$$$. At first, all stacks are rigidly registered to masked $$$X^{init}$$$ for global alignment. This is followed by global 3D FFD registration of $$$X^{init}$$$ to all stacks. At the first DSVR iteration ($$$q=0$$$), the global FFD transformations {$$$G_{l}^{D}$$$} are used for initialisation of FFD BSpline registration of $$$X^{init}$$$ to slices {$$$Y_{k}$$$} producing {$$$T_{k}(q)$$$} transformations. The SR reconstruction is used for iterative recovery of high resolution volume, and is performed similarly to the rigid case [3], except that the point spread function needs to be non-rigidly deformed. The output $$$X(q)$$$ volume is then used in the next FFD DSVR loop. The interleaved DSVR SR steps are repeated for $$$Q=3$$$ iterations with gradual refinement of BSpline grid spacing: $$$d(q)=$$${15; 10; 5}mm identified as optimal for fetal body dimensions. This constraint prevents overfitting to motion corrupted features of $$$X^{init}$$$. The structure-based outlier rejection is based on structural similarity maps between {$$$Y_{k}$$$} and X(q) transformed with {$$$T_{k}(q)$$$}. DSVR was implemented based on MIRTK library [9] as a part of SVRTK package [10].The method was evaluated on fetal iFIND [7] T2-weighted MRI datasets. The iFIND acquisitions were performed on a 1.5T MRI using ssFSE: TR=15000ms, TE=80ms, voxel size 1.25x1.25x2.5mm, slice thickness 2.5mm and spacing 1.25mm. The stacks have different orientations with respect to the fetal trunk and uterus.
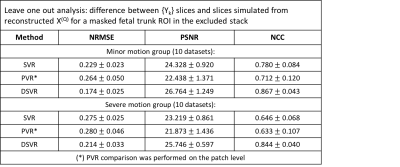
The datasets selected for quantitative evaluation included 20 cases from 28-31 weeks GA range each containing 6 stacks. The cases were divided into two groups with respect to severity of motion (visually assessed by an operator). Taking into account the absence of the ground truth, we employed classical leave one out approach [3] when one of the stacks is excluded from SR reconstruction. The difference between the original {$$$Y_{k}$$$} and slices simulated from $$$X(q)$$$ is assessed in terms of intensity and structural similarity metrics computed in the masked trunk ROI of the excluded stack.
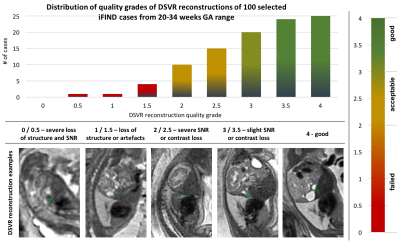
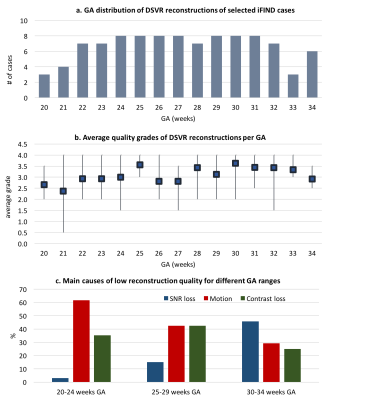
An additional qualitative assessment of DSVR was performed on randomly selected 100 datasets from 20-34 weeks GA (Fig.5.a).
Results
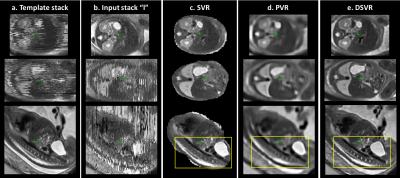
Quantitative assessment: DSVR, SVR [3] and PVR [5] reconstructions were performed for each of the selected 20 datasets. Fig.2. shows an example of the results for a minor motion case. DSVR reconstructed volumes are characterised by well-defined features and texture of fetal body organs in comparison to the other methods. The quantitative results (Fig.3) for intensity and structural metrics demonstrate that DSVR outperforms SVR and PVR for both severe and minor motion datasets.Qualitative assessment: The DSVR reconstructed volumes for 100 iFIND cases were graded by clinicians trained in fetal MRI with respect to image quality in [0; 4] range (4 corresponding to high quality and <2 to failed). Fig.4 presents distribution of the number of cases per grade and examples of different image quality. The average grades per GA (Fig.5.b) vary within 2.5-3.5 range (3.09±0.78). Only 6% of all cases failed (scored <2), due to large rotations and bending that could not be resolved by FFD registration. The identified main causes of lower grades are motion for younger cases and low SNR for older cases (Fig.5.c).
Conclusions
Quantitative evaluation of DSVR showed that it outperforms both SVR and PVR methods for the task of fetal body reconstruction and resolves non-rigid deformations of organs. Furthermore, it showed consistently good reconstruction quality for 100 datasets from a wider GA range. However, due to limitation of the classical registration methods the current implementation of DSVR is not designed for correction of large amplitude motion outside the capture range of gradient-descent based FFD registration. This will be addressed in future by advanced registration methods [11,12].Acknowledgements
The iFIND project data used in this research were collected subject to the informed consent of the participants. This work was supported by the NIH Human Placenta Project grant [1U01HD087202-01], the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z), the Wellcome Trust and EPSRC IEH award [102431] for the iFIND project and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
[1] N. C. Nowlan, “Biomechanics of foetal movement,” European Cells and Materials, vol. 29, pp. 1–21, 2015.
[2] A. Gholipour, J. A. Estroff, and S. K. Warfield, “Robust super-resolution volume reconstruction from slice acquisitions: Application to fetal brain MRI,” IEEE Transactions on Medical Imaging, vol. 29, no. 10, pp.1739–1758, 2010.
[3] M. Kuklisova-Murgasova, G. Quaghebeur, M. A. Rutherford, J. V. Hajnal, and J. A. Schnabel, “Reconstruction of fetal brain MRI with intensity matching and complete outlier removal,” Medical Image Analysis, vol. 16, no. 8, pp. 1550–1564, 2012.
[4] D. F. A. Lloyd, K. Pushparajah, J. M. Simpson, J. F. van Amerom, M. P. M. van Poppel, A. Schulz, B. Kainz, M. Deprez, M. Lohezic, J. Allsop, S. Mathur, H. Bellsham-Revell, T. Vigneswaran, M. Charakida, O. Miller, V. Zidere, G. Sharland, M. Rutherford, J. Hajnal, and R. Razavi, “Three-dimensional visualisation of the fetal heart using prenatal MRI with motion corrected slice-volume registration.” The Lancet, no. 10181, pp. 1619–1627.
[5] A. Alansary, M. Rajchl, S.G. McDonagh, M. Murgasova, M. Damodaram, D. F. Lloyd, A. Davidson, M. Rutherford, J. V. Hajnal, D. Rueckert, and B. Kainz, “PVR: Patch-to-Volume Reconstruction for Large Area Motion Correction of Fetal MRI,” IEEE Transactions on Medical Imaging, vol. 36, no. 10, pp. 2031–2044, 2017.
[6] A. Uus, T. Zhang, L. Jackson, M. Rutherford, J. V. Hajnal, and M. Deprez, “Deformable Slice-to-Volume Registration for Respiratory Motion Correction in Abdominal and In-utero MRI,” in ISMRM 2019, 2019.
[7] “iFIND Project.” [Online]. Available: http://www.ifindproject.com/. [Accessed: 01-Nov-2019].
[8] D. Rueckert, L. I. Sonoda, C. Hayes, D. L. G. Hill, M. O. Leach, and D. J. Hawkes, “Nonrigid Registration Using Free-Form Deformations: Application to Breast MR Images,” IEEE Transactions on Medical Imaging, vol. 18, no. 8, pp. 712–721, 1999.
[9] “MIRTK: Medical Image Registration ToolKit.” [Online]. Available: https://github.com/BioMedIA/MIRTK. [Accessed: 01-Nov-2019].
[10] “SVRTK: MIRTK based SVR package.” [Online]. Available: https://github.com/SVRTK/SVRTK. [Accessed: 01-Nov-2019].
[11] S. S. Salehi, S. Khan, D. Erdogmus, and A. Gholipour, “Real-Time Deep Pose Estimation With Geodesic Loss for Image-to-Template Rigid Registration,” IEEE Transactions on Medical Imaging, vol. 38, no. 2, pp. 470–481, 2019.
[12] G. Balakrishnan, A. Zhao, M. R. Sabuncu, J. Guttag, and A. V. Dalca, “VoxelMorph: A Learning Framework for Deformable Medical Image Registration,” IEEE Trans. Med. Imaging, vol. 38, no. 8, pp. 1788–1800, 2019.
Figures