0458
Free-Breathing Abdominal Magnetic Resonance Fingerprinting Using a Pilot Tone Navigator1Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Physics, Case Western Reserve University, Cleveland, OH, United States
Synopsis
This study presents a novel free-breathing technique which addresses some of the difficulties in quantitative T1 and T2 mapping of the abdomen. The technique integrates Magnetic Resonance Fingerprinting (MRF) and pilot tone (PT) navigator to retrospectively provide simultaneous quantification of multiple tissue properties in the abdomen in inhalation and expiration states of the respiratory motion. The proposed method can be implemented with both 2D and 3D MRF acquisitions.
Introduction
Quantitative T1 and T2 mapping in the abdomen can provide insight into diseased and healthy tissue characterization for aiding lesion detection. However, quantitative T1 and T2 mapping of the abdomen can be immensely challenging due to the effects of respiratory motion. MR Fingerprinting (MRF) is a relatively new framework for quantitative MR imaging. Previous studies have shown that it can provide simultaneous 2D T1 and T2 quantification in a single breath-hold for abdominal imaging[1]. While MRF holds great potential for tissue characterization, its use in the thorax and abdomen has been hindered by the requirement for long breath-holds. Previous studies have shown that navigator based free-breathing MRF is feasible, but the temporal resolution of the navigator is relatively low[2]. Here, we present a completely self-navigated pilot tone (PT) navigator[3-5] based free-breathing MRF technique that achieves high temporal resolution and can be implemented in both 2D and 3D coverage of the abdomen while simultaneously quantifying multiple tissue properties.Methods
Overview of the method is shown in Figure1. Similar to [3-4], PT signal is transmitted from a function generator (Rigol DG4162) through an in-house built loop antenna inside the MR suite waveguide. The function generator is synchronized to the 10 MHz clock of the 3T Skyra. The PT frequency is set to be outside the region of interest at the edge of the FOV. For the spiral MRF acquisitions used here, this meant outside of the bandwidth that included the imaging FOV (approx. 100kHz from the center frequency). PT signal varies corresponding to physical motion and is encoded in the raw data through receiver arrays (TIM body 18). Principal component analysis is applied to pilot tone signals from all coils. Navigator signal was chosen from the principal component that showed the most relative power in the frequency band of the respiratory motion, roughly 0.14-0.5 Hz. The temporal resolution of the pilot tone signal is determined by sequence repetition time which is largely improved from the previous method (6.1ms vs 300ms)[2].The details of the 2D MRF sequence for abdominal imaging have been introduced in[1]. This initial implementation used purely retrospective gating. To this end, 10 measurements, 17280 time-points, of a coronal slice are acquired to ensure sufficient respiratory data has been gathered. The total acquisition time is 3 minutes. In the future, this will be completely prospective, and thus almost as efficient as a breath-holding (BH) exam. Imaging parameters included: FOV, 400x400 mm; matrix, 256x256; slice thickness, 5mm. As in the previous method[1], a MRF dictionary is generated using the Bloch Simulation[6] with all combinations of signal evolution with T1(100-3000ms) and T2(10-500ms). To evaluate the performance of PT signal, a respiratory belt is used while collecting 2D PT MRF data. The resulting respiratory signal is aligned based on scan timing to the PT signal.
By applying a threshold on the navigator waveform, subset data can be selected for various respiratory phases. In this study, we extracted the end-inhalation and end-exhalation states by binning all the time points within the threshold together. 3000 time-points in each respiratory state were selected to represent a fully sampled MRF sequence. A simple pattern matching algorithm is performed on the subset image series with the corresponding dictionary and trajectory. At the end of this process, the spiral sampling distribution will be non-uniform due to the nature of PT binning, therefore spiral density is compensated based on the occurrence of each spiral arm.
The 3D MRF sequence is adapted from the literature[7]. Four measurements are repeated for the navigator to generate enough data to form a complete dataset in each respiratory state. The imaging parameters are 400x400 mm; matrix, 256x256; partition thickness, 3mm; number of partitions, 24. Total scan time for 4 repetitions was ~20 minutes. Data from the 4 measurements are extracted to create a complete 3D dataset in each respiratory state after binning. Image reconstruction for 3D MRF dataset is accelerated using SVD dictionary compression[8]. Five normal volunteers participated in this study, and T1, T2 values are extracted from liver, kidney, spleen, and muscle using ROI analysis.
Results
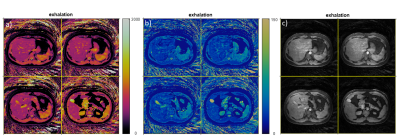
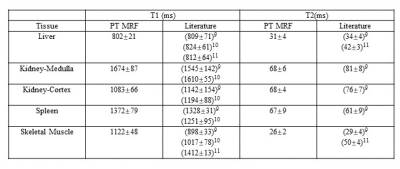
The PT navigator waveform was validated against the signal acquired using a respiratory belt in Figure2. 2D free-breathing results are presented in Figure3. Figure4 shows the 3D maps acquired from another volunteer in the axial view.A summary of T1 and T2 relaxation times for 5 subjects acquired with the 3D MRF technique is presented in table1.
Discussion and Conclusion
This proof-of-concept study shows the feasibility of PT Navigator based 2D- and 3D-MRF technique for quantitative free-breathing abdominal imaging. The 2D case can simultaneously generate quantitative maps in each respiratory state in 3 minutes, with potential for reduced scan time, and the results are comparable to breath-hold scans. Further improvement in threshold selection method could allow better binning of data in both the 2D and 3D methods. In the future, this method is expected to enable a completely prospective scan protocol, which should have dramatically increased efficiency by more uniformly sampling both the respiratory and k-space encoding dimensions. In summary, our results show that self-navigated MRF approaches hold great potential to provide simultaneous quantification of multiple tissue properties in the abdominal free-breathing acquisition.Acknowledgements
This material is supported by Siemens Healthcare and the National Science Foundation Graduate Research Fellowship Grant No. CON501692.References
[1]. Chen Y, et al. Magnetic resonance fingerprinting (MRF) for rapid quantitative abdominal imaging. Intl. Soc. Mag. Reson. Med. 23: 561.
[2]. Chen Y, et al., 2016. Free-Breathing 3D Abdominal Magnetic Resonance Fingerprinting using Navigators. Intl. Soc. Mag. Reson. Med. 24: 716.
[3]. Schroeder L, et al., 2016. Two-Dimensional Respiratory-Motion Characterization for Continuous MR Measurements Using Pilot Tone Navigation. In Proc. Intl. Soc. Mag. Reson. Med. 24:310.
[4]. Speier S, et al., 2015. PT-Nav: A Novel Respiratory Navigation Method for Continuous Acquisition Based on Modulation of a Pilot Tone in the MR-Receiver. ESMRMB 129:97-98, 2015. Doi: 10.1007/s10334-015-0487-2.
[5]. Ludwig J, et al,. 2019. Pilot tone-based prospective respiratory motion correction for 2D cine cardiac MRI. In Proc. Intl. Soc. Mag. Res. Med. 27: 73.
[6]. Ma, D. et al. Magnetic Resonance Fingerprinting. Nature 495, 187–192 (2013).
[7]. Chen, Y. et al. Three-dimensional MR Fingerprinting for Quantitative Breast Imaging. Radiology 290, 33–40 (2018).
[8]. McGivney, D. F. et al. SVD Compression for Magnetic Resonance Fingerprinting in the Time Domain. IEEE Trans Med Imaging 33, 2311–2322 (2014).
[9]. de Bazelaire, CMJ., MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230, 652–659 (2004).
[10]. Chen, Y. et al. Rapid volumetric t1 mapping of the abdomen using three-dimensional through-time spiral GRAPPA. Magnetic Resonance in Medicine 75, 1457–1465 (2016).
[11]. Stanisz, G. J. et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic Resonance in Medicine 54, 507–512 (2005).
Figures