0457
Motion Compensation in Pulmonary Ultra-short Echo Time MRI: Preliminary results in Idiopathic Pulmonary Fibrosis1Dept. of Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Dept. of Radiology and Biomedical Imaging, University of California - San Francisco, San Francisco, CA, United States, 3UCSF/UC Berkeley Graduate Program in Bioengineering, University of California - San Francisco, San Francisco, CA, United States, 4Dept. of Medicine, University of Wisconsin - Madison, Madison, WI, United States, 5Dept. of Radiology, University of Wisconsin - Madison, Madison, WI, United States, 6Dept. of Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Acquiring pulmonary MRI images without motion corruption is a challenging task. In this work, we evaluate several conventional and advanced retrospective motion compensation techniques in subjects with idiopathic pulmonary fibrosis (IPF). We evaluate the effectiveness of each technique using concomitantly acquired CT scans, contrast to noise, and sharpness measures. We find that registration-based techniques show a significant improvement in CNR and sharpness. We also observe significantly improved image quality when referenced side-by-side with CT. We conclude that registration-based techniques could be used to better resolve subtle fibrotic textures in IPF.
Introduction
Idiopathic Pulmonary Fibrosis (IPF) is a fatal disease that affects approximately 5 million people worldwide.1 Diagnosis of this disease is often made using a combination of high-resolution computed tomography (HRCT) and histology. The pathology of IPF has been difficult to characterize using gold standard pulmonary function tests (PFTs), and progression is difficult to evaluate with current clinical tools. Histology is an invasive technique, and concerns about radiation dose limit the applicability of HRCT for longitudinal monitoring. Ultrashort echo-time (UTE) magnetic resonance imaging has previously shown to provide similar clinical information to HRCT in various diseases in adults and neonates with the added benefit of not using ionizing radiation.2–5 However, due to the longer acquisition times, motion corruption is frequently observed in UTE images. The purpose of this study was to evaluate alternative motion correction/compensations strategies in patients with interstitial fibrotic lung disease with the goal of identifying a current best approach for visualizing lung parenchymal texture using UTE MRI.Methods
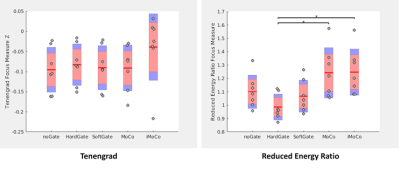
Optimized UTE datasets were selected from a larger ongoing HIPAA-compliant and IRB approved study to evaluate IPF. Selection was done by using an automated texture analysis on CT scans acquired on the same visit, and the 7 subjects with the lowest percentages of normal lung were selected for preliminary analysis. Four retrospective motion compensation techniques were considered: 1) hard thresholding of the bellows signal to 50% (hard-gating), 2) exponentially weighting of the bellows signal (soft-gating)6, 3) motion-state resolved compressed sensing reconstruction with non-rigid image registration and averaging (MoCo), and 4) an iterative motion compensation reconstruction technique (iMoCo).7 For iMoCo, we fixed the number of motion states to 6 and the regularization parameter to λTGV = 0.025 across all subjects. We evaluated the quality of the different approaches using a few methods. First, we evaluate the different techniques qualitatively and compare them directly to HRCT images. For a quantitative analysis, regions of interest were manually drawn in homogeneous regions in the liver, lung parenchyma, aorta, and airways. We evaluate the contrast to noise ratio (CNR) of these regions (relative to the airways) for each method. We then evaluate image sharpness using two different measures: The Tenengrad focus measure - Sobel filtering and calculation of the mean of resulting directional gradient images in the superior/inferior direction; and the Reduced Energy Ratio (RER) - frequency decomposition using the discrete cosine transform (DCT) and taking the ratio of the energy of the first 512 coefficients normalized to the DC coefficient.8 We chose these methods because a) IPF subjects are known to have severe fibrosis predominantly in the basal and posterior portions of the lungs, which could obfuscate a region of interest sharpness measure, e.g. in the diaphragm, and b) it is desirable to have a metric that could quantify overall sharpness of the image, including the lung fibrosis itself. Although both metrics rely on high frequency information for their results, the reduced energy ratio should be more robust to noise due to the exclusion of small high frequency components.Results
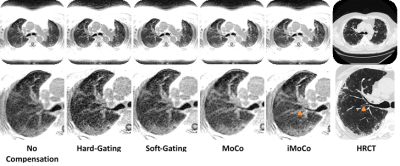
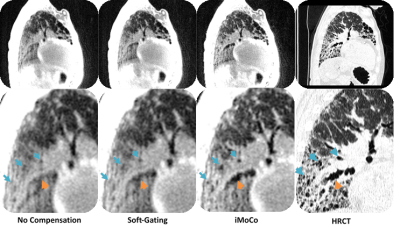
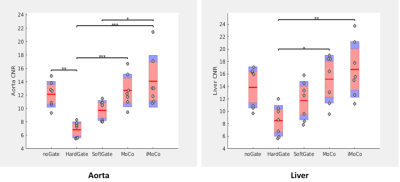
After compensating for motion using conventional gating techniques, it is clear that a lot of texture is lost when compared to the corresponding CT (Fig. 1). Registration-based motion compensation techniques MoCo and iMoCo have the best visual appearance of the MRI based motion compensation techniques, and compare most favorably with the corresponding CT. Specifically, iMoCo resolves texture more clearly and even begins to resolve some traction bronchiectasis lucency (orange arrow) that is seen only on CT. In a case of severe honeycombing (Fig. 2), iMoCo shows significant improvement in texture contrast when compared to other techniques. Bronchiectasis (orange arrow) and honeycombing (blue arrows) are better resolved on iMoCo than other techniques. Quantitative measures of CNR and sharpness support the qualitative trends (Figs. 3 and 4), however, we note that the lung parenchyma CNR did not show any trends (not shown). iMoCo shows higher CNR in both structures and is statistically significant compared to conventional soft gating in the aorta (p < 0.05) and hard gating in the aorta (p < 0.001) and liver (p < 0.01). The sharpness measure RER indicates that iMoCo resolves more texture than conventional hard-gating (p < 0.05). Tenengrad also suggests higher sharpness in iMoCo, but statistical significance was not reached with our sample size.Discussion
Preliminary results suggest a significant improvement using registration-based motion compensation techniques. iMoCo clearly depicts more texture in fibrotic disease compared to conventional motion compensation strategies. iMoCo and MoCo trend towards higher CNR in the aorta and the liver hinting that they might be able to resolve subtler tissue differences than the other methods, however lung parenchyma CNR did not show improvement. This could be due to over regularization in the compressed sensing reconstructions causing excessive smoothing in areas of inherently low signal.Conclusion
Initial assessment of advanced motion compensation strategies suggest that significant improvement can be made over conventional gating techniques by using registration-based techniques such as MoCo and iMoCo. While promising, further work needs to be done to optimize choice of regularization to further improve these techniques for characterizing fibrotic lung disease.Acknowledgements
The authors thank our collaborators and colleagues. This work was supported by NIH/NHLBI grants R01 HL126771, R01 HL136965, UL1TR000427 to University of Wisconsin Institute for Clinical and Translational Research (ICTR), and the University of Wisconsin Pulmonary Imaging Center (NIH S10 OD016394). This project was also supported in part through a fellowship to Luis Torres from the University of Wisconsin Science and Medicine Graduate Research Scholars Program (SciMed GRS).References
1. Meltzer, E. B. & Noble, P. W. Idiopathic pulmonary fibrosis. Orphanet J. Rare Dis. 3, 8 (2008).
2. Benlala, I. et al. Automated Volumetric Quantification of Emphysema Severity by Using Ultrashort Echo Time MRI: Validation in Participants with Chronic Obstructive Pulmonary Disease. Radiology 292, 216–225 (2019).
3. Roach, D. J. et al. Morphological and quantitative evaluation of emphysema in chronic obstructive pulmonary disease patients: A comparative study of MRI with CT. J. Magn. Reson. Imaging 44, 1656–1663 (2016).
4. Ohno, Y. et al. Pulmonary high-resolution ultrashort TE MR imaging: Comparison with thin-section standard- and low-dose computed tomography for the assessment of pulmonary parenchyma diseases. J. Magn. Reson. Imaging 43, 512–532 (2016).
5. Higano, N. S. et al. Quantification of Neonatal Lung Parenchymal Density via Ultrashort Echo Time MRI With Comparison to CT. J. Magn. Reson. Imaging JMRI 46, 992–1000 (2017).
6. Johnson, K. M., Block, W. F., Reeder, S. B. & Samsonov, A. Improved least squares MR image reconstruction using estimates of k-space data consistency. Magn. Reson. Med. 67, 1600–1608 (2012).
7. Zhu, X., Chan, M., Lustig, M., Johnson, K. M. & Larson, P. E. Z. Iterative motion-compensation reconstruction ultra-short TE (iMoCo UTE) for high-resolution free-breathing pulmonary MRI. Magn. Reson. Med. 0,.
8. Lee, S.-Y., Yoo, J.-T., Kumar, Y. & Kim, S.-W. Reduced Energy-Ratio Measure for Robust Autofocusing in Digital Camera. IEEE Signal Process. Lett. 16, 133–136 (2009).
Figures