0456
3D Self-Navigator Acquisition for Translational and Nonrigid Motion Correction in Multiphase Coronary MR Angiography1Stanford University, Stanford, CA, United States
Synopsis
A whole-heart multiphase coronary angiography method has been developed that extracts a 3D navigator image every heartbeat from the 3D cones high-resolution imaging data. Such 3D self-navigators (sNAVs) enable direct beat-to-beat respiratory motion tracking of the heart for translational and nonrigid correction. 3D sNAVs are derived from the inner k-space region of phyllotaxis-ordered 3D cones interleaves collected over multiple cardiac phases. A multiscale low-rank method is used for reconstruction.
Introduction
A free-breathing whole-heart coronary magnetic resonance angiography (CMRA) method based on a 3D cones k-space trajectory has been developed1. To monitor respiratory motion, a 3D image-based navigator (iNAV) is acquired every heartbeat during diastole, immediately after the primary imaging sequence. 3D iNAVs enable beat-to-beat tracking and compensation of translational and nonrigid respiratory motion of the heart. However, a time delay exists between the primary image acquisition and the navigator acquisition. In this work, we propose the acquisition of a 3D self-navigator (sNAV); that is, a 3D navigator image derived every heartbeat from the primary imaging sequence itself. Such an acquisition removes the delay between the primary imaging and the navigator data sequence. It also frees up the sequence to image multiple cardiac phases.Sequence Design
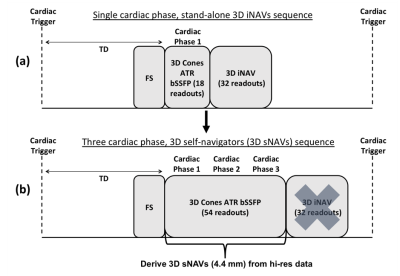
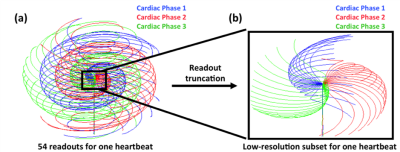
Figure 1 shows the CMRA sequences for both stand-alone 3D iNAVs and 3D sNAVs. In both sequences, an alternating-TR (ATR) balanced steady-state free precession (bSSFP) sequence is used to obtain high-resolution data with a 3D cones trajectory. In the proposed sequence, high-resolution data is collected over three cardiac phases and an sNAV is reconstructed directly from the inner k-space region of those high-resolution readouts (Figure 1b).For the high-resolution sequence, a segment of 18 cone interleaves is acquired every heartbeat for each cardiac phase2,3. To avoid unwanted eddy-current effects in the bSSFP acquisition, a modified phyllotaxis reordering technique4 is used (Figure 2a). While phyllotaxis reordering provides a more distributed k-space coverage compared to a standard sequential collection pattern, the low-resolution k-space subset (i.e., the central portion of the trajectory) for deriving a 3D sNAV still exhibits notable gaps (Figure 2b), creating significant aliasing artifacts in the sNAV if gridding reconstruction is used. Therefore, we iteratively solve for the beat-to-beat sNAVs by exploiting parallel imaging and multiscale low-rank (MLR) constraints5. Prior work has demonstrated that by partitioning undersampled navigator images into blocks of a fixed size and applying a low-rank constraint over the temporal domain in compressed sensing reconstructions, aliasing artifacts are alleviated, and accurate motion measurements can be obtained6. A limitation of this approach is that it assumes respiration dynamics are only correlated across time with blocks of one fixed size. The MLR reconstruction technique addresses this limitation by promoting low-rank constraints across different block sizes over time. As such, we adapt this approach for reconstruction of the undersampled 3D sNAVs. The overall reconstruction formulation is a generalization of both locally low-rank + sparse models and hence, retains their advantages7.
Experimentation
To assess the method’s ability to correct for rigid-body motion, a phantom scan with stepwise motion was done with a sequence that combined both the three-phase acquisition for the sNAV and the stand-alone 3D iNAV acquisition. The scanner table was oscillated by up to 30 mm in the z-direction. The resulting translational estimates from the iNAVs and sNAVs, as well as the associated motion-corrected phantom images, were examined. To study the method’s ability to correct for nonrigid motion, scans were performed on volunteers using the same combined sNAV/iNAV sequence, and motion-corrected with autofocusing8. Volunteers with sufficiently low heartrates (<55 bpm) were scanned to allow acquisition of both the sNAV and stand-alone 3D iNAV for comparison. The sequence was implemented on a 1.5T GE scanner using an 8-channel cardiac array. Image parameters were 28 x 28 x 14 cm3 FOV and 1.2 mm isotropic resolution. The ATR-bSSFP parameters were TE/TR1/TR2 = 0.57/4.29/1.15 ms and tip angle = 70°. Scan time was 610 heartbeats.Results
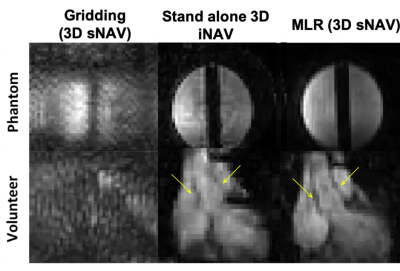
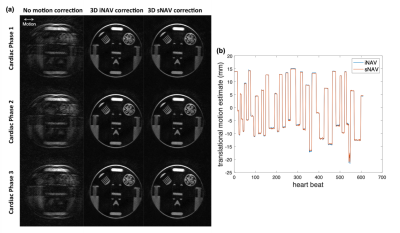
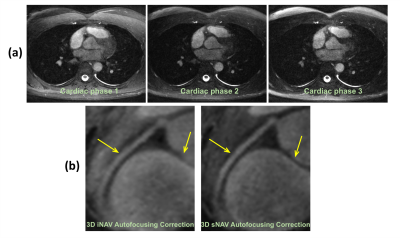
Figure 3 shows phantom and volunteer study results comparing 3D iNAVs to 3D sNAVs. The MLR reconstruction simultaneously suppresses aliasing artifacts and recovers structural information as compared to the sNAVs reconstructed with gridding. Although both navigators exhibit 4.4 mm isotropic resolution, somewhat better depiction is achieved with sNAVs.Results of the moving phantom study are given in Figure 4. 3D sNAVs and 3D iNAVs yield nearly identical translationally motion-corrected images for all three cardiac phases (Figure 4a). This is supported by the close correspondence in the superior-inferior (z-direction) motion estimates obtained from both iNAVs and sNAVs (Figure 4b).
Figure 5a shows an example of axial images from all three cardiac phases that were motion-corrected using 3D sNAVs. An example of a right coronary artery image (Figure 5b) reveals improved sharpness in the image motion-corrected using sNAVs.
Conclusion
In this work, we demonstrated an acquisition method based on 3D cones for 3D multiphase coronary imaging with 3D navigator images extracted from the imaging data. To facilitate this method, a modified phyllotaxis readout ordering technique was used to distribute the k-space sampling in each heartbeat. Furthermore, a multiscale low-rank method was used to reconstruct beat-to-beat 3D sNAVs directly from the imaging data. This enabled imaging across multiple cardiac phases and removed the delay between the acquisition of the primary imaging data and the navigator data.Acknowledgements
NIH HL127039, GE Healthcare, NSF GRFP, Knight-Hennessy Scholars ProgramReferences
1. Addy NO, Ingle RR, Luo J, et al. 3D image-based navigators for coronary MR angiography: Coronary 3D Image-Based Navigators. Magn Reson Med. 2017;77(5):1874-1883. doi:10.1002/mrm.262692.
2. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006;55(3):575-582. doi:10.1002/mrm.207963.
3. Wu HH, Gurney PT, Hu BS, Nishimura DG, McConnell MV. Free-breathing multiphase whole-heart coronary MR angiography using image-based navigators and three-dimensional cones imaging: 3D Cones Coronary MRA. Magn Reson Med. 2013;69(4):1083-1093. doi:10.1002/mrm.243464.
4. Malavé MO, Baron CA, Addy NO, et al. Whole‐heart coronary MR angiography using a 3D cones phyllotaxis trajectory. Magn Reson Med. 2019;81(2):1092-1103. doi:10.1002/mrm.274755.
5. Ong F, Lustig M. Beyond Low Rank + Sparse: Multiscale Low Rank Matrix Decomposition. IEEE J Sel Top Signal Process. 2016;10(4):672-687. doi:10.1109/JSTSP.2016.25455186.
6. Jiang W, Ong F, Johnson KM, et al. Motion robust high resolution 3D free-breathing pulmonary MRI using dynamic 3D image self-navigator: Motion Robust High-Resolution 3D Pulmonary MRI. Magn Reson Med. 2018;79(6):2954-2967. doi:10.1002/mrm.269587.
7. Otazo R, Candès E, Sodickson DK. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components: L+S Reconstruction. Magn Reson Med. 2015;73(3):1125-1136. doi:10.1002/mrm.252408.
8. Luo J, Addy NO, Ingle RR, et al. Nonrigid Motion Correction With 3D Image-Based Navigators for Coronary MR Angiography: Nonrigid Motion Correction for Coronary MRA. Magn Reson Med. 2017;77(5):1884-1893. doi:10.1002/mrm.26273
Figures