0453
Motion corrected reconstruction of abdominal SWEEP data using local similarity graphs and deformable slice to volume registration1Biomedical Engineering, School of Biomedical Engineering & Imaging Sciences, Kings College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering & Imaging Sciences, Kings College London, London, United Kingdom, 3Department of Forensic and Neurodevelopmental Science, Institute of Psychiatry, Psychology & Neuroscience, Kings College London, London, United Kingdom, 4Department of Women and Children’s Health, School of Life Course Sciences, Kings College London, London, United Kingdom
Synopsis
In this work we introduce a novel pipeline for motion correction of SWEEP style acquisition data. The method utilizes local similarity graphs for efficient generation of static volumes by extracting the most coherent slices within a local neighborhood and interpolating over missing data. These static volumes are then used as registration targets for a patch-based deformable slice-to-volume registration. The pipeline produces highly coherent 3D volumes and is demonstrated in adult abdominal and fetal/placental imaging using 2D SWEEP bSSFP and SPGR acquisitions.
Introduction
SWEEP is an approach to imaging that efficiently acquires densely sampled M2D stacks for short TR, steady-state sequences by continuously advancing the excited slice location at each excitation.[1] A key application domain is where there is subject motion such that rapid slice acquisition is able to freeze in-plane motion but inter-slice motion prevents 3D assessment of images. For correction of rigid motion slice-to-volume registration (SVR) methods can be deployed.[2] However, when applied to abdominal and in utero imaging, SVR approaches produce incorrect results due to non-rigid deformation of tissues from multiple sources of motion.An initial approach for motion correction of abdominal SWEEP data used variation in body area as a surrogate for respiratory state to allow creation of homogeneous subgroups of slices that could be interpolated onto a regular grid to create registration targets for eventual non-rigid alignment of all data.[1] A better approach would be to recognise that respiration leads to both spatially and temporally variable displacements and seek to focus on local similarity in space and time. In this work we demonstrate effective motion correction of abdominal SWEEP data using this strategy combined with a patch based deformable slice-to-volume registration (DSVR).[3]
Methods
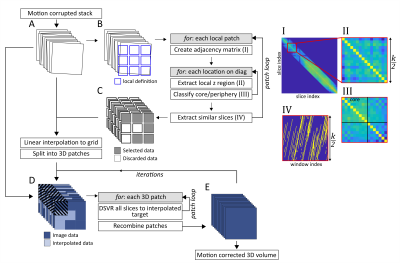
Imaging was performed on a Philips 3T Achieva system equipped with a 32-channel cardiac coil. Informed consent was obtained from 10 pregnant volunteers (Gestational age (GA): 25-32w) and 2 non-pregnant adults. Both angiography and structural imaging was performed with relevant acquisition parameters given in table 1.An outline of the motion correction process is illustrated in Figure 1. The image volume is assessed in the context of overlapping local patches which defines the local in-plane region. For each of these patches an adjacency matrix is created, weighted by the normalised cross-correlation with corresponding patches in its $$$k$$$ nearest neighbour slices.
Finding the subset of slice patches that optimizes the similarity over a region can then be formulated as a graph connectivity problem, where the slices that are most similar over a local region will have the most highly weighted connections. A sliding window with $$$k/2$$$ slices was moved along the diagonal of the adjacency matrix, clustering was performed within each window to classify slices as “core” and “periphery”. Core-periphery classification was performed by maximising similarity between core slices while minimising the similarity between peripheral slices for each window. [4] The core-ness resolution parameter ($$$\gamma$$$) defines the relative split between core/periphery classifications. A suitable value was found by maximizing $$$γ$$$ under the constraint that the greatest gap in selected slices is equal to the acquisition slice thickness. Slices that were frequently selected across multiple neighbouring windows were then considered to be core slices across the volume and used to create a local interpolation mask.
Every in-plane ($$$xy$$$) location was then linearly interpolated in the slice direction ($$$z$$$) using the subset of slices from the nearest similarity patch to create a volume with minimal inter-slice motion artifacts. This volume was used as a registration target for a patch-based DSVR registration process where all slices, regardless of classified motion state were registered into the same space. This new volume could then be fed back as a new, more accurate, registration target for an iterative reconstruction approach. This resulted in a full FOV motion corrected volume that efficiently utilized all acquired data.
Results
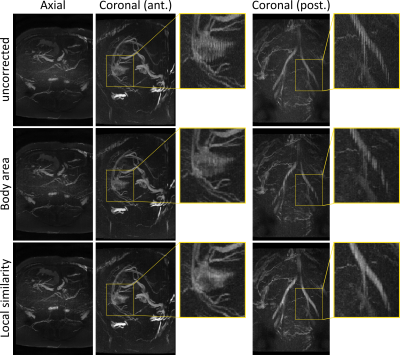
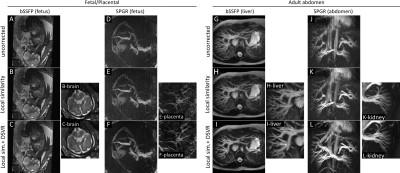
A comparison between the previously described body area method and the proposed local similarity graph method is shown in figure 2. The body area method corrects for respiration displacement only, and performs well in anterior regions where this is the primary source of motion. When other sources of motion are present, or in regions where the respiration surrogate is a poor match to local motion the body area method discards valid data creating an inconsistent volume. The proposed method performs better in these regions since it accommodates rather than eliminates other sources of motion.Figure 3 shows some example reconstructions from the full pipeline. Examples from 4 individual volunteers are shown, including bSSFP and SPGR examples for both fetal/placental and adult abdominal imaging, exhibiting the wide applicability of the motion correction pipeline. Each example in Figure 3 also includes views from other parts of the motion corrected volumes showing different anatomy demonstrating the value of a full 3D motion corrected volume.
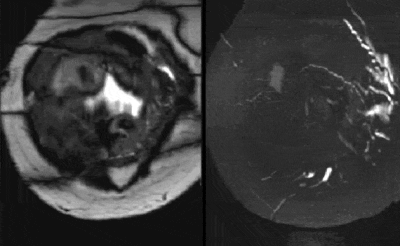
Figure 4 shows two fully motion corrected volumes acquired in a pregnant volunteer imaged at 37 weeks. In this case separate bSSFP and SPGR acquisitions were performed sequentially providing detailed angiography and matching anatomical context. Both the image volumes show highly coherent data demonstrating effective motion correction.
Discussion
The described motion correction pipeline is shown to be effective for removing respiratory and other motion artifacts from SWEEP data. The quality of the SVR methods are highly dependent on the quality of the initial registration target. In this case the novel use of a local similarity graphs to generate an initial registration target produces better results than the previous method.Conclusion
The described methods provide effective motion compensation for respiratory corrupted abdominal SWEEP acquisitions. These techniques have been demonstrated for both fetal/placental and adult abdominal imaging cases.Acknowledgements
This work was supported by the NIH Human Placenta Project grant 1U01HD087202-01 (Placenta Imaging Project (PIP)), the Wellcome Trust (Sir Henry Wellcome Fellowship, 201374/Z/16/Z), the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z) and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
[1] Jackson, L. H., et al. "Respiration resolved imaging with continuous stable state 2D acquisition using linear frequency SWEEP." Magnetic resonance in medicine (2019).
[2] Kuklisova-Murgasova, M., Quaghebeur, G., Rutherford, M. A., Hajnal, J. V. & Schnabel, J. A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 16, 1550–1564 (2012).
[3] Uus, Alena, et al. "Deformable Slice-to-Volume Registration for Motion Correction in Fetal Body MRI." arXiv preprint arXiv:1906.08827 (2019).
[4] Rubinov, Mikail, et al. "Wiring cost and topological participation of the mouse brain connectome." Proceedings of the National Academy of Sciences 112.32 (2015): 10032-10037.
Figures