0452
Bulk Motion Compensated Image Reconstruction for Renal Function Estimation with DCE-MRI1Radiology, Boston Children's Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Urology, Boston Children's Hospital, Boston, MA, United States
Synopsis
Dynamic Radial VIBE (DRV) DCE-MRI can provide high spatio-temporal resolution in the dynamic series of volumes used to evaluate the kidney function. However, bulk motion during the scan corrupts the volumes and deteriorates the quality of the kidney function estimation. We introduce a bulk-motion robust image reconstruction technique to mitigate the effects of motion. Our algorithm detects corrupted k-space data, reconstructs the volumes without signal dropout and aligns them. We applied this approach on non-sedated babies undergoing feed-and-wrap DCE-MRI with DRV. Our results show that our method improves the image quality and the estimation of the kidney function parameters.
Introduction
Babies with antenatally detected hydronephrosis need to be evaluated for kidney anatomy and function to determine if they would benefit from surgical intervention to prevent permanent loss of kidney function. Kidney function is evaluated with measures of glomerular filtration rate (GFR) and differential renal function (DRF) per kidney. Dynamic contrast enhanced MRI using a motion-robust radial VIBE technique and feed and wrap imaging can provide GFR and DRF measures for babies without sedation [1], [2]. Dynamic Radial VIBE (DRV) sequence is capable of providing the required high spatio-temporal resolution necessary to capture fast dynamics of contrast concentration in the aorta after bolus injection of contrast for accurate tracer kinetic model fitting. We can then estimate the filtration rate parameter and compute the GFR and DRF markers [3]. However, when infants move during DCE-MRI acquisition, volumes in the dynamic series acquired during motion events become corrupted. These volumes cannot be reacquired because of inability to repeat contrast-injection. Motion events also severely deteriorate the quality of the tracer-kinetic model fitting to compute clinical parameters. We introduce a bulk-motion robust, image reconstruction technique to mitigate the effects of motion. We first detect k-space data acquired during motion events, which are compromised, and discard them. Then, we use temporal regularization term in the reconstruction to fill the information for the discarded k-space lines during image reconstruction. We also realign the misaligned volumes due to motion events to obtain a correctly aligned dynamic series of volumes.Methods
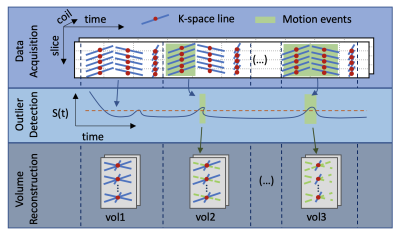
We acquired radial VIBE DCE-MR images from 11 infants, where 4 infants had no bulk motion, 3 had moderate motion and 4 had severe motion. Infants were imaged after being fed, swaddled and rocked to sleep, without sedation. The images were acquired with a “stack-of-stars” 3D FLASH prototype sequence using a multi-channel body-matrix coil (3T Siemens Skyra/Trio, TR/TE/FA 3.56/1.39ms/12o, 32 coronal slices, voxel size=1.25x1.25x3mm, 1326 radial spokes acquired in 6 mins with golden angle radial ordering). Our motion robust image reconstruction algorithm is composed of three steps: 1) Detection of the presence of bulk motion using the center of the k-space lines of each slice (i.e. the center point of the k-space data in each slice, where kx,ky=0). We computed an outlier/motion metric [4] that measures the average cross correlation (CC) between each FID and those within a neighboring temporal window of 3s. A drop in the value of this CC metric indicates a motion event. We identified the stacks of k-space lines corrupted by motion as having an outlier metric (1-CC) greater than one standard deviation above the median throughout the entire scan and ignored them during reconstruction. 2) Motion-robust reconstruction after removal of corrupted k-space lines: We used the remaining k-space lines to reconstruct the sequence of volumes using iterative GRASP reconstruction with a temporal regularization constraint [3], [5]. Each volume was reconstructed using 34 lines per slice (~3.3 s/volume) minus those corrupted by motion. 3) Alignment of the resulting sequence of volumes affected by motion: We registered each volume to a reference volume selected as the one which has the smallest outlier metric averaged across all k-space lines used for its reconstruction. To evaluate our approach, we compare the proposed motion-compensated image reconstruction (MoCo) with the standard image reconstruction (GRASP) using the entire set of k-space data. We compared the average edge strength (AES) of the sequences of volumes reconstructed with both methods. We also fitted a tracer kinetic (TK) model to the average contrast agent concentration over time per kidney [6]. For each subject, we repeated the TK model over 100 wild bootstrap sampling iterations [7] and computed the goodness of fit as the normalized root-mean-squared error (nRMSE).Results
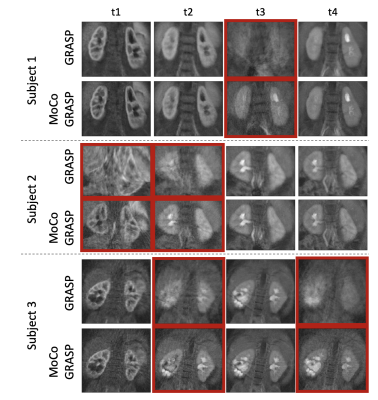
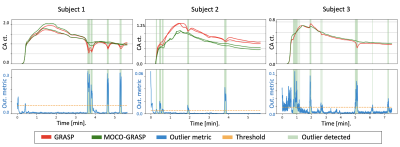
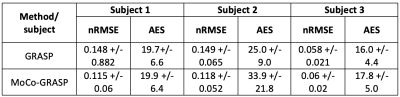
We computed the proposed motion metric and applied the MoCo-GRASP reconstruction on 3 subjects with moderate/intermittent motion. The subjects with no motion are reconstructed without outlier removal with standard GRASP and subjects with severe/continuous motion did not have sufficient uncorrupted k-space lines for reconstruction. Figure 2 compares the volumes reconstructed with standard GRASP and the proposed MoCo-GRASP. The GRASP reconstructions present large motion artifacts resulting in signal dropout and blurring of the images acquired during motion events. In contrast, MoCo-GRASP compensates for most of the signal dropout and reduces the blurriness of the images reconstructed. The signal dropout is also observed in the concentration curves obtained from the standard GRASP reconstructions (Figure 3). These signal dropout events match with increases in the outlier metric, identified as motion events. On the other hand, the concentration curves of the MoCo-GRASP reconstructions are smoother and do not present large signal loss. The numerical results are summarized in Table 1. These show an average increase in AES of 3.6 +/- 6.5 and a reduction in nRMSE of 0.014 +/- 0.02.Conclusions
We presented a motion-compensated image reconstruction method that is robust to outliers generated due to sudden motion. The volumes reconstructed with the novel method do not present signal dropout and blurring during motion events. Consequently, the concentration curves do not have large outliers and the goodness of fit of the tracer-kinetic models improves. This method will potentially enable non-sedated DCE-MR imaging of babies with hydronephrosis for evaluation of their kidney function.Acknowledgements
This work was supported partially by the Boston Children's Hospital Translational Research Program Pilot Grant 2018, Society of Pediatric Radiology Multi-center Research Grant 2019, Crohn’s and Colitis Foundation of America’s (CCFA) Career Development Award and AGA-Boston Scientific Technology and Innovation Award 2018 and by NIDDK of the National Institutes of Health under award number R01DK100404.References
[1] S. Kurugol, O. Afacan, C. Seager, R. S. Lee, J. S. Chow, and S. K. Warfield, “Compensating for Bulk Motion in Feed and Wrap Renal Dynamic Radial VIBE DCE-MRI using Bulk Motion Removal and Non-Rigid Registration,” Int. Soc. Magn. Reson. Med., no. Mc, pp. 72–74, 2018.
[2] C. Seager et al., “Feed and Wrap Magnetic Resonance Urography Provides Anatomic and Functional Imaging in Infants Without Anesthesia,” J. Pediatr. Urol., 2019.
[3] L. Feng et al., “Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI.,” Magn. Reson. Med., vol. 72, no. 3, pp. 707–17, Sep. 2014.
[4] J. Coll-Font et al., “Self- navigated bulk motion detection for feed and wrap renal dynamic radial VIBE DCE- MRI,” in International Society for Magnetic Resonance in Medicine (ISMRM), 2019.
[5] K. T. Block et al., “Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity,” J. Korean Soc. Magn. Reson. Med., vol. 18, no. 2, p. 87, 2014.
[6] S. P. Sourbron, H. J. Michaely, M. F. Reiser, and S. O. Schoenberg, “MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model,” Invest. Radiol., vol. 43, no. 1, pp. 40–48, 2008.
[7] R. Davidson and E. Flachaire, “The wild bootstrap, tamed at last,” J. Econom., vol. 146, no. 1, pp. 162–169, Sep. 2008.
Figures