0451
Bias Field Correction in Hyperpolarized 129Xe Gas Ventilation MRI1Medical Physics Graduate Program, Duke University, Durham, NC, United States, 2Biomedical Engineering, Duke University, Durham, NC, United States, 3Radiology, Duke University, Durham, NC, United States
Synopsis
The presence of RF inhomogeneity in hyperpolarized 129Xe ventilation MRI affects quantitative analysis and interpretation. These inhomogeneities are commonly corrected using the N4ITK algorithm, which retrospectively calculates a smoothly varying bias field with non-uniform intensity normalization. However, this algorithm has not been rigorously validated for functional imaging. Here we show, using flip angle maps derived directly from 3D-radial acquisitions of ventilation, that N4ITK may over-correct bias field and remove inherent physiological gradients. We illustrate this comparison by a combination of simulation, phantom, and in vivo ventilation imaging.
Introduction
Hyperpolarized (HP) 129Xe MRI is increasingly used for 3D visualization of regional pulmonary ventilation1. Since these images are often acquired using close-fitting transmit/receive coils, their associated B1 inhomogeneity (“bias field”) leads to regional variations in both flip angles and detection sensitivity. These factors must be corrected to achieve robust and repeatable quantitative image analysis. The current de facto standard for such bias field correction is currently N4ITK2, which retrospectively calculates a smoothly varying bias field through iteratively sharpening the high frequency content of the intensity distribution3. However, this technique has never been validated for lung MRI, where inherent physiological gradients may be mistakenly removed. To address these questions, we compare the bias fields and corrected ventilation images using N4ITK to the recently proposed approach of mapping flip angles directly from 3D radial imaging4. Using a combined approach of simulations, phantom, and in vivo imaging, we demonstrate that N4ITK bias field correction likely needs further attention as we seek to interpret more than defects from 129Xe ventilation MRI.Methods
Digital Phantom Simulations Simulations of N4ITK versus flip angle mapping were performed using a digital Shepp-Logan Phantom(matrix=256x256), which was Fourier-transformed to generate k-space data and subjected to RF-induced magnetization decay, ignoring T1 effects. K-space was modulated with a simulated, smoothly varying bias field, with flip angles regionally varying between 2 and 7o. The data was radially sampled with 1000 radial spokes (128 points/spoke), using a 2-D golden-angle pattern. Images were reconstructed using a conjugate-gradient nonuniform FFT technique5. These were then corrected using both N4ITK and the flip angle mapping technique of Niedbalski4. N4ITK was applied using default B-spline fitting parameters and a shrink factor of 2 over a mask defining the phantom edge. The flip angle map was generated using the ratio of images reconstructed with the first and second half of k-space. This was converted to a bias field estimate of both transmit and receive sensitivity using $$B1\propto\alpha* \sin(\alpha)*(1-cos^{n_{views}}(\alpha))/(1-\cos(\alpha))$$ and smoothed with an approximating B-spline6 (spline order=3; number of levels=4x4x4; number of control points=4x4x4).Phantom Imaging This analysis was extended to a system with a known, uniform 129Xe distribution affected only by magnetization decay. Isotopically enriched 129Xe gas was polarized to 25% in volumes of 700 mL and delivered to an evacuated 43-liter acrylic cylinder (h=61cm, D=30cm). The cylinder was wrapped with water tubes to provide coil loading and enable proton localizers. This assembly was wrapped with a quadrature flexible 129Xe transmit-receive chest coil. 129Xe imaging was performed with a 3.0T Siemens Magnetom Trio Scanner (VB19) using a randomized 3D Halton spiral radial sequence (views=1000; samples/view=128; TR/TE=7.5/0.46ms; flip angle=2o; FOV=50cm). 129Xe images were reconstructed using a kernel sharpness7 of 0.15 onto 128x128x128 matrices. Images were bias field corrected using both methods and constrained with a mask defining the phantom volume. Corrected images were rescaled to the 99th percentile such that intensities ranged from 0-1 for comparison.
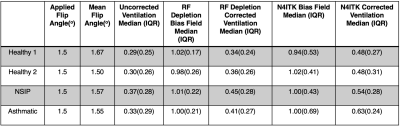
In Vivo Imaging Five subjects (3 healthy, 1 young asthmatic, 1 interstitial pneumonia) underwent radial ventilation imaging (views=3600; TR/TE=4.5/0.45ms; flip angle=1.5o; FOV=40cm) in the supine position. One healthy subject was imaged both supine and prone. Images were bias field corrected using both methods as described above, with T1 assumed to be uniform within the lung.
Results
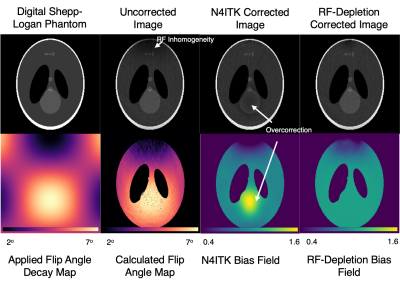
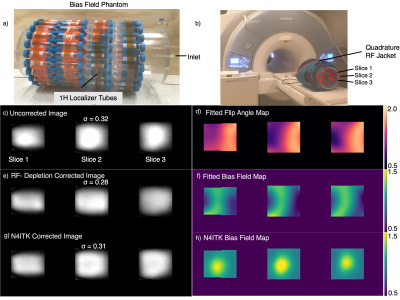
Figure 1 depicts the digital phantom, applied bias field, and uncorrected and corrected images. Note the N4ITK approach misinterprets the bright feature in the phantom as bias field while the flip-angle approach more appropriately corrects for RF inhomogeneity. However, there are residual regions of intensity variations attributable to regions of relatively low SNR in areas of low flip angle.Figure 2 illustrates imaging, flip angle maps, and the two types of correction for the cylindrical 129Xe spin-density phantom. Flip angle maps can also be extracted in phantoms since T1 is long with respect to imaging time. Standard deviation calculations of spin density inside the masked region demonstrate that both bias field correction methods decrease image heterogeneity compared to the uncorrected value ($$$\sigma_{uncor}=0.32,\sigma_{cor-RF}=0.28,\sigma_{cor-N4ITK}=0.31$$$). However, the two methods generate very different spatial patterns.
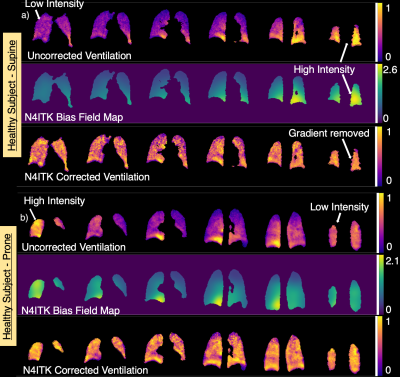
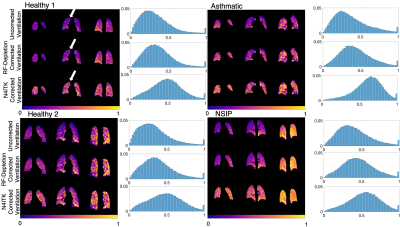
Figure 3 shows ventilation imaging, N4ITK-derived bias field, and bias-corrected imaging in a healthy subject scanned both prone and supine. This study illustrates how physiological ventilation gradients in the anterior-posterior (AP) direction may be incorrectly filtered out with N4ITK. Figure 4 shows radially acquired ventilation maps and distribution histograms in 4 subjects that consistently demonstrate that N4ITK applies a stronger intensity correction than is indicated by the RF-depletion approach. This is confirmed by the quantitative metrics in Figure 5.
Discussion
Digital phantom simulations and in-vivo imaging demonstrate that physiological ventilation gradients can be suppressed with standard N4ITK bias field correction as compared to corrections derived from flip angle mapping. These flip angle maps are uniquely derived from radial acquisitions4, but not from the more commonly employed GRE imaging. While these overcorrections are unlikely to materially affect calculation of ventilation defect percentages, they could have a more significant effect on analyzing other aspects of the ventilation distribution such as high and low-intensity voxels. Thus, for imaging that seeks to study and preserve physiological gradients, bias field correction based on radially acquired flip angle maps should be favored.Acknowledgements
R01HL105643, R01HL12677, NSF GRFP DGE-1644868References
1. Petousi, N., Talbot, N. P., Pavord, I. & Robbins, P. A. Measuring lung function in airways diseases: Current and emerging techniques. Thorax 797–805 (2019). doi:10.1136/thoraxjnl-2018-212441
2. Song, S., Zheng, Y. & He, Y. A review of Methods for Bias Correction in Medical Images. Biomed. Eng. Rev. 3, 1–10 (2017).
3. Tustison, N. J., Cook, P. A. & Gee, J. C. N4ITK: Improved N3 Bias Correction. 29, 1310–1320 (2011).
4. Niedbalski, P. J. et al. Mapping and correcting hyperpolarized magnetization decay with radial keyhole imaging. Magn. Reson. Med. 82, 367–376 (2019).
5. Lin, J. M. Python non-uniform fast fourier transform (PyNUFFT): An accelerated non-cartesian MRI package on a heterogeneous platform (CPU/GPU). J. Imaging 4, 1–22 (2018).
6. Tustison, N. J. & Gee, J. C. N-D Ck B-Spline Scattered Data Approximation. 1–13 (2006).
7. Robertson, S. H. et al. Optimizing 3D noncartesian gridding reconstruction for hyperpolarized 129 Xe MRI-focus on preclinical applications. Concepts Magn. Reson. Part A 44, 190–202 (2015).
Figures