0440
19F-MRI of inhaled perfluoropropane for assessment of pulmonary ventilation: a multi-centre reproducibility study in healthy volunteers1Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom, 2Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom, 3POLARIS, Academic Radiology, University of Sheffield, Sheffield, United Kingdom, 4Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom, 5Institute of Health and Society, Newcastle University, Newcastle upon Tyne, United Kingdom
Synopsis
19F-MRI of inhaled perfluoropropane can be used to assess regional pulmonary ventilation. We conducted a prospective multi-centre reproducibility study in 40 healthy volunteers. Same-day static breath-hold 19F-MR images with 1 cm isotropic resolution were acquired on four occasions for each volunteer following inhalation of a perfluoropropane/oxygen gas mixture. Percentage ventilated lung volume (%VV) was calculated for all volunteers, reflecting the inhalation protocol, imaging protocol, and image registration and segmentation process applied. Volunteer %VV was determined to within ±1.7% (95% CI). Gas inhalations were well tolerated by all volunteers with no adverse events.
Introduction
Existing clinical imaging modalities that facilitate regional assessment of respiratory disease (such as CT and V/Q scintigraphy) necessitate exposure to ionising radiation, restricting serial use. MRI of exogenous gas agents offers a safely-repeatable alternative for the assessment of regional pulmonary ventilation. Hyperpolarized noble gas MRI of inhaled 3He and 129Xe has undergone considerable refinement in recent years, providing high signal-to-noise ratio (SNR) images within a single breath-hold duration.1-2 The technique can provide quantitative and clinically useful metrics of pulmonary function, such as the percentage ventilated lung volume (%VV), which has proven clinical efficacy in patients with lung disease.3 However, the specialist equipment and expertise required for the hyperpolarization process presents a potential barrier to widespread clinical application.19F-MRI of thermally polarized inhaled perfluoropropane (PFP, C3F8) is an emerging approach to human ventilation imaging. Recent studies have demonstrated the feasibility of assessing regional pulmonary ventilation in healthy volunteers and patients with respiratory disease.4-7 PFP gas can be used directly from the cylinder, supplied as a 79% PFP/21% oxygen (PFP/O2) gas mixture, with no technical preparation required. Moreover, its short longitudinal relaxation time (~10ms) permits a high degree of signal averaging. However, its relatively scarce signal and short in vivo T2* (~2ms)4 presents technical challenges in achieving image quality approaching that of hyperpolarized noble gas MRI.
In this work, we performed a multi-centre reproducibility study in 40 healthy volunteers using 19F-MRI of inhaled PFP to assess the reproducibility of %VV measurements, in preparation for clinical studies of patients with respiratory disease.
Methods
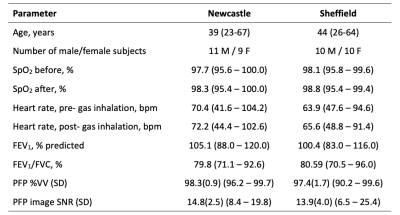
Ethical approval was granted by the Newcastle and North Tyneside 1 Research Ethics Committee (Ref 16/NE/0282) and the NHS Health Research Authority. 40 healthy volunteers were recruited and scanned at one of two research sites (Sheffield: 10M, 10F, aged 26-64 years; Newcastle: 11M, 9F, aged 23-67 years). All volunteers were non-smokers with no history of pulmonary disease. Screening was performed immediately before commencing the MR scan session, including a spirometry test to confirm normal lung function.Images were acquired using a 19F/1H birdcage torso coil (Rapid Biomedical, Rimpar, Germany) at each site built to the same specifications and interfaced to a Philips 3T Achieva scanner (Newcastle) or 3T Ingenia scanner (Sheffield). Each volunteer performed three deep inhalations (to ~total lung capacity) of the PFP/O2 gas mixture followed by a breath-hold (13.5s), during which a 3D SPGR 19F-MR imaging sequence (TE=1.7ms, TR=7.5ms, flip angle=45°, FOV=400×(310-360, volunteer size dependent)×250mm3, resolution=10×10×10mm3, bandwidth=500Hz/pixel, averages=3, acquisition time=13.5s) was acquired. Gas inhalation and 19F-MRI were acquired a total of 4 times per volunteer, each separated by at least a 5-minute interval to ensure complete gas wash-out prior to commencement of the following acquisition. 3D 1H anatomical images (TE=0.49ms, TR=4ms, flip angle=6°, FOV=440×440×247.5mm3 resolution=3×3×7.5mm3, bandwidth=3400Hz/pixel, acquisition time=14.6s) were acquired during separate breath-holds. Heart rate and oxygen saturation levels were monitored in all volunteers throughout the MR scan session, and for a further 10 minutes upon leaving the scanner.
Registration and segmentation were performed using in-house software developed in Matlab,8 built around ITK-SNAP (v.3.6.0, USA).9 Anatomical 1H images were registered to 19F-MR ventilation images to correct for any differences in depth of breath-hold between acquisitions. The registered 1H and 19F lung images were then segmented independently by two trained assessors using a semi-automated thresholding-based process to determine total lung volume (1H) and ventilated lung volume (19F), from which %VV was determined. %VV was assessed by fitting the variance component for the differences between volunteers, between acquisitions, and between observers. Inter-assessor agreement was also evaluated by computing the Dice Similarity Coefficient (DSC) of the segmented ventilation images.
Results
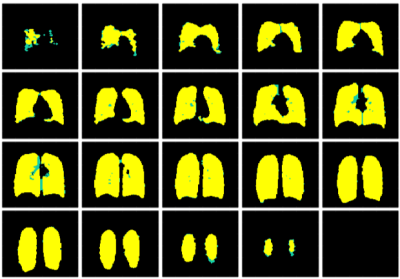
19F-MRI was well-tolerated by all volunteers with no adverse events. Table 1 lists site-specific volunteer metrics. 2/40 healthy volunteers were excluded from data analysis owing to poor compliance with the breathing protocol. Figure 1 shows coronal slices from a 3D 19F-MRI of PFP distribution in the lungs of a typical healthy volunteer. Average 19F scan SNR (standard deviation, SD) across the remaining 38 volunteers was 14.4(3.3). The mean measured %VV (SD) was 97.8(1.3), with determination of a volunteer correct to ±1.7% (95% CI), accounting for variance between acquisitions and inter-assessor variation. A high mean DSC (SD) of 0.97(0.02) was achieved. The corresponding segmentations made by two independent assessors are displayed in Figure 2, with regions of agreement (yellow) and discrepancies (green) visible.Discussion
19F images were acquired from 38 healthy volunteers with image quality and SNR sufficient for determination of %VV. High inter-rater reliability was achieved across all datasets, with minor discrepancies mainly localised to the anterior periphery of the lung, where decreased coil sensitivity is apparent. Our work demonstrates technical feasibility for collecting reproducible datasets at different independent research sites, in preparation for multi-centre studies involving patients with respiratory disease. The gas was well tolerated by all volunteers, adding weight to the growing body of evidence surrounding safe use of inhaled PFP/O2 for human ventilation imaging.Conclusion
This study assessed the reproducibility of 19F-lung ventilation MRI methods and determined the capability and limitations of this imaging technique for mapping regional pulmonary ventilation. MR imaging of a cohort of 80 patients with obstructive lung disease (COPD and asthma) is currently underway using these scan methods.Acknowledgements
This work was supported by a Medical Research Council Developmental Pathway Funding Scheme grant (MR/N018915/1).
We gratefully acknowledge Matthew Clemence (Philips Medical Systems) for his advice and expertise, and the work of the MR radiographers at the Newcastle Magnetic Resonance Centre and the Sheffield Teaching Hospitals NHS Foundation Trust.
References
1. Kirby M, Pike D, Coxson HO, et al. Hyperpolarized 3He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology. 2014;273(3):887-896.
2. Marshall H, Horsley A, Taylor CJ, et al. Detection of early subclinical lung disease in children with cystic fibrosis by lung ventilation imaging with hyperpolarised gas MRI. Thorax. 2017;72(8):760-762.
3. Wild J, Smith L, Horn F, et al. Hyperpolarised gas MR lung imaging – Breaks through to clinical practice. European Respiratory Journal. 2015;46(59):OA4992.
4. Couch MJ, Ball IK, Li T, et al. Pulmonary ultrashort echo time 19F MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: Feasibility. Radiology. 2013;269(3):903-909.
5. Halaweish AF, Moon RE, Foster WM, et al. Perfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans, Chest. 2013;144(4):1300-1310.
6. Neal MA, Pippard BJ, Hollingsworth KG, et al. Optimized and accelerated 19F-MRI of inhaled perfluoropropane to assess regional pulmonary ventilation. Magnetic Resonance in Medicine. 2019;0(0):1-11.
7. Gutberlet M, Kaireit TF, Voskrebenzev A, et al. Free-breathing dynamic 19F gas MR imaging for mapping of regional lung ventilation in patients with COPD. Radiology. 2018;286(3):1040-1051.
8. Hughes PJ, Horn FC, Collier GJ, et al. Spatial fuzzy c‐means thresholding for semiautomated calculation of percentage lung ventilated volume from hyperpolarized gas and 1H MRI. J. Magn. Reson. Imaging. 2018;47:640-646.
9. Paul A. Yushkevich, Joseph Piven, Heather Cody Hazlett, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006;31(3):1116-28.
Figures