0431
Quantitative dose-dependent changes in regional lung function after radiation therapy detected using xenon-129 gas exchange MRI1Center for In Vivo Microscopy, Duke University, Durham, NC, United States, 2Department of Radiation Oncology, University of North Carolina, Chapel Hill, NC, United States, 3Department of Radiation Oncology, Duke University Medical Center, Durham, NC, United States
Synopsis
Radiation therapy (RT) is widely used to treat lung cancer, but damage to surrounding healthy tissues can lead to compromised lung function. In this study, patients undergoing RT were imaged pre- and post-treatment using hyperpolarized 129Xe gas exchange MRI to assess for RT-induced changes in regional lung function. At 3-months post-treatment, a dose-response was evident in ventilation and gas exchange. Lung regions receiving ≥20Gy exhibited significantly increased barrier uptake and decreased RBC transfer. This may help radiation oncologists further understand the dose-dependence of RT-induced lung injury, and design dose distributions with fewer treatment toxicities.
Introduction
Approximately 100,000 US patients per year undergo radiation therapy (RT) for lung cancer. While RT improves tumor control and survival, it also risks causing radiation-induced lung injury1 (RILI). Approximately 5-50% of patients who receive conventional thoracic RT develop acute clinically significant radiation pneumonitis (RP), presenting as fever, congestion, persistent cough, and dyspnea; an estimated 2% of these patients die2,3. Subsequent late effects can manifest as lung fibrosis, permanently reducing pulmonary function and quality of life. Currently, predicting whether a specific patient’s treatment plan will cause significant lung injury is challenging4, due in part to the inability to regionally measure damage to lung tissue. The ability to map and quantify such functional changes as a function of radiation dose could enable better prediction and prevention of RILI. To address this, we obtained pre- and post-RT hyperpolarized (HP)-129Xe gas exchange MRI in patients being treated for non-small cell lung cancer (NSCLC).Methods
Six patients receiving conventional RT for NSCLC underwent breath-hold HP-¹²⁹Xe gas exchange MRI. Subjects were imaged pre- and 3-months post-RT, with two subjects also imaged at 6-months post-RT. As previously established5,6, gas exchange MRI exploits the solubility and chemical shift of ¹²⁹Xe to permit 3D imaging of its distribution into the lung airspaces (ventilation), uptake in the interstitial barrier tissues, and its transfer to red blood cells (RBC). A breath-hold 3D radial 1H image is also acquired to confine analysis to the thoracic cavity. The gas-phase image is normalized by its top 1% of signal to map regional ventilation; voxel-wise signal ratios of 129Xe barrier/gas map interstitial barrier uptake, while RBC/gas measures end-to-end function (RBC transfer). The patients’ RT planning CT scans were registered to the pre-treatment anatomical ¹H MRI using a commercial deformable algorithm (RaySearch Laboratories, Stockholm, Sweden). This allows the radiation dose distribution to be transferred into the MR frame of reference. Each post-treatment MRI was rigidly registered to the subject’s pre-treatment MRI to enable calculation of functional change on a per-voxel basis. Spatial registration uncertainty was partly accounted for by smoothing the functional images using a Gaussian filter (FWHM=6.25mm). Voxels excluded from analysis were those with high dose gradient (≥3Gy/mm), those receiving ≥95% of prescription dose, and those with ventilated xenon signal <2σnoise above background. Voxels receiving similar dose (within 5Gy) were binned and data from all subjects was combined to plot radiation dose vs. mean functional change for ventilation, barrier uptake, and RBC transfer. Functional changes at 3-months were compared in lung receiving <20Gy vs ≥20Gy using paired t-tests, where 20Gy represents a standard threshold for potential lung damage in conventional lung RT1.Results
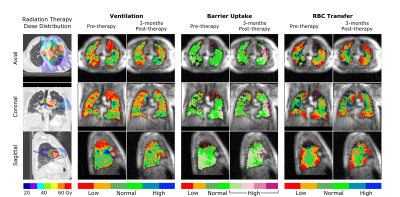
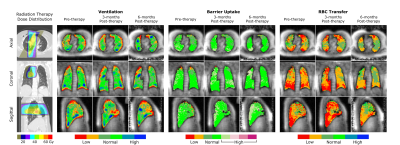
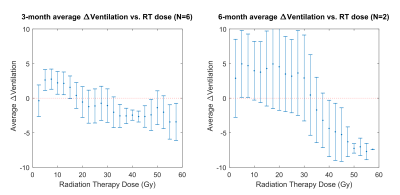
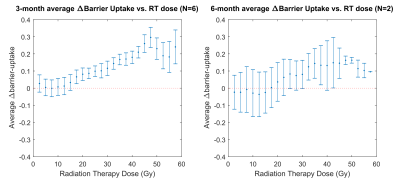
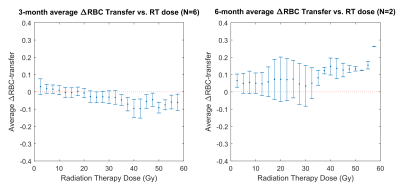
Figures 1 and 2 show two representative patients with their dose distributions and functional change maps. Key features include: prominently increased barrier uptake in the left lung of Figure 1; mildly increasing barrier uptake at both post-RT time points in Figure 2; and complex behavior of the RBC transfer in Figure 2 (an apparent decrease at 3-months, followed by an increase at 6-months). Across the patient ensemble, Figure 3 demonstrates that ventilation decreases with dose for both time-points. At 3-months, average relative ventilation increased by 1.6% in <20Gy voxels and decreased by 2% in the ≥20Gy voxels (P<0.08). Figure 4 shows that above a ~15-20Gy threshold, barrier uptake increases at both 3- and 6-months. At 3-months, average barrier uptake in ≥20Gy voxels increased by 27%, compared to just 3% in <20Gy voxels (P<0.02; percentages represent the change in barrier/gas divided by the healthy reference mean barrier/gas). The effect of RT on RBC transfer was more complex, showing a decrease at 3-months above ~35-40Gy, but increasing in high-dose regions at 6-months (Figure 5). At 3-months, RBC transfer decreased by 7% in ≥20Gy voxels while increasing by 2% in <20Gy voxels (P<0.03; percentages are again relative to the healthy reference mean).Discussion
The observed increase in barrier uptake is consistent with the known inflammatory nature of early stage RILI2. Similarly, decreasing RBC transfer at 3-months for high-dose voxels suggests reduced regional function. Previous studies using nuclear medicine approaches demonstrated dose-dependent reductions in regional perfusion and, to a lesser degree, regional ventilation.7 The magnitude and volume of these reductions were related to decline in global lung function8 (pulmonary function tests and symptoms). Our dose-response trend in 129Xe-MRI ventilation agrees with this previous work. We note that the 129Xe-MRI measures of barrier uptake and RBC transfer are new and unique metrics that have not been previously investigated in RT patients. Although they do not measure the exact same physiological process, our 3-month trend for RBC transfer agrees with prior perfusion studies; however, our 6-month results deviated from the expected change in perfusion.Conclusion
Hyperpolarized-¹²⁹Xe MRI detects RT-induced, dose-dependent changes in regional lung function. Future work, in concert with prior perfusion and ventilation studies, may enhance our understanding of the physiology of RT-induced lung injury. These methods could be applied to larger cohorts and longitudinal studies, relating the observed functional changes to global lung function decline and RILI symptoms. In addition, understanding the regional functional sensitivity of lung tissue to radiation could inform RT treatment planning optimization9,10 and reduce the rate of RT-associated lung injury.Acknowledgements
This work was supported in part by National Institutes of Health (NIH) grant R01HL105643References
1. Marks LB, Bentzen SM, Deasy JO, et al. Radiation Dose–Volume Effects in the Lung. Int J Radiat Oncol. 2010;76(3):S70-S76.
2. Mehta V. Radiation pneumonitis and pulmonary fibrosis in non–small-cell lung cancer: Pulmonary function, prediction, and prevention. Int J Radiat Oncol. 2005;63(1):5-24.
3. Ohe Y. Treatment-related death from chemotherapy and thoracic radiotherapy for advanced cancer. Panminerva Med. 2002;44(3):205-212.
4. Das SK, Zhou S, Zhang J, et al. Predicting Lung Radiotherapy-Induced Pneumonitis Using a Model Combining Parametric Lyman Probit With Nonparametric Decision Trees. Int J Radiat Oncol. 2007;68(4):1212-1221.
5. Wang Z, Robertson SH, Wang J, et al. Quantitative analysis of hyperpolarized 129Xe gas transfer MRI. Med Phys. 2017;44(6):2415-2428.
6. Kaushik SS, Robertson SH, Freeman MS, et al. Single-breath clinical imaging of hyperpolarized 129Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med. 2016;75(4):1434-1443.
7. Boersma LJ, Damen EMF, de Boer RW, et al. Dose-effect relations for local functional and structural changes of the lung after irradiation for malignant lymphoma. Radiother Oncol. 1994;32(3):201-209.
8. Boersma LJ, Damen EMF, de Boer RW, et al. Estimation of overall pulmonary function after irradiation using dose-effect relations for local functional injury. Radiother Oncol. 1995;36(1):15-23.
9. McGuire SM, Zhou S, Marks LB, et al. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol. 2006;66(5):1543-1552.
10. Yamamoto T, Kabus S, Bal M, et al. The first patient treatment of computed tomography ventilation functional image-guided radiotherapy for lung cancer. Radiother Oncol. 2016;118(2):227-231.
Figures