0430
Benchmarking acinar airway measurements from inhaled nanoparticles with hyperpolarized 129Xe diffusion-weighted MRI1Academic Radiology, University of Sheffield, Sheffield, United Kingdom, 2Division of Ergonomics and Aerosol Technology, Lund University, Lund, Sweden, 3Department of Translational Medicine, Lund University, Malmö, Sweden
Synopsis
Airspace Dimension Assessment with inhaled nanoparticles (AiDA) and hyperpolarized 129Xe diffusion-weighted (DW)-MRI was performed in twenty-three healthy volunteers to benchmark measurements from AiDA against those from 129Xe DW-MRI. Significant correlations were observed between AiDA derived root mean square distal airspace radius, and 129Xe apparent diffusion coefficient and diffusion model derived acinar airway dimensions. Furthermore, the AiDA recovery at zero-second breath-hold significantly correlated with 129Xe alpha index from the stretched exponential model, a marker of acinar airspace heterogeneity. This benchmarking study demonstrates the potential of AiDA as an alternative method for the clinical evaluation of acinar airway microstructure changes.
Introduction
Hyperpolarized 129Xe diffusion-weighted (DW)-MRI provides 3D in-vivo information of lung microstructure with the derived apparent diffusion coefficient (ADC) due to Brownian gas atom diffusion restriction within the acinar airways. Theoretical models of gas diffusion within the lungs, such as the cylinder airway model (CM)1 and stretched exponential model (SEM)2, are used to derive in-vivo acinar airways dimensions in patients with a range of pulmonary diseases3.Airspace Dimension Assessment with inhaled nanoparticles (AiDA) is a recently proposed alternative non-invasive technique for evaluating distal airspace dimensions with measurements of nanoparticles deposited by Brownian diffusion in the distal airways4. AiDA measures the recovery of inhaled nanoparticles after a series of breath-holds of varying duration, and analysis yields an estimated root mean square distal airspace radius (rAiDA) and the recovery at zero-second breath-hold (R0 intercept)5. In healthy volunteers, rAiDA significantly correlated with MRI proton lung tissue density6, but further benchmarking with established methods of in-vivo acinar airspace assessment is required. The aim of this work was therefore to benchmark AiDA measurements against 129Xe DW-MRI derived ADC and acinar airway dimensions in a healthy volunteer cohort.
Methods
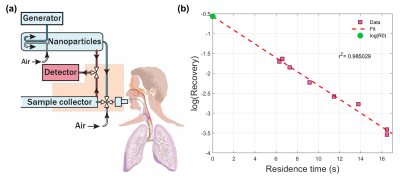
Twenty-three healthy volunteers (14M, 9F) with no history of lung disease were recruited for AiDA at Lund University, and hyperpolarized 129Xe DW-MRI at University of Sheffield. This study was approved by the Regional Ethical Review Board in Lund, Sweden, and performed in accordance with the Declaration of Helsinki, including obtaining informed written consent from all volunteers.A schematic illustrating the instrument used for AiDA is shown in Figure 1a5. Nanoparticles were generated by aerosolizing polystyrene latex nanospheres using an electrospray, size selected (50 nm) using a differential mobility analyzer and diluted with particle free air. The inhaled and exhaled nanoparticle concentration was continuously sampled using a condensation particle counter for different consecutive breath-hold times of 5, 5, 6, 8, 10, 12, 15, and 15 seconds. The nanoparticle residence time in the lungs was found by adding an individual’s breathing phase to the breath-hold times. An exponential decay curve was fitted to recovery values for each residence time (Figure 1b) to derive rAiDA from the half-life (t1/2) of this decay and the diffusion coefficient (D)4: $$$\text{r}_{\text{AiDA}}=\sqrt{Dt_{1/2}}$$$. R0 intercept was subsequently derived from the extrapolation of the exponential decay to a theoretical recovery value at zero-second residence time.
Hyperpolarized 129Xe DW-MRI was acquired on a GE HDx 1.5T scanner with a flexible transmit/receive quadrature vest coil using a 3D multiple b-value SPGR sequence with compressed sensing2. DW-MRI acquisition parameters were: diffusion time=8.5 ms, b=[0, 12, 20, 30 s/cm2]. 129Xe ADC was calculated on a voxel-by-voxel basis using a mono-exponential fit between b=0 and 12 s/cm2 interleaves; while for 129Xe acinar airway dimensions, the SEM2 and CM1 was fitted to all four b-values to derive: mean diffusive length scale (LmD), alpha heterogeneity index, acinar airway radius (RCM) and mean chord length (Lm) measurements. Correlations between inhaled nanoparticles and global 129Xe DW-MRI measurements were assessed using Spearman’s rank correlation tests in GraphPad Prism 8.
Results and Discussion
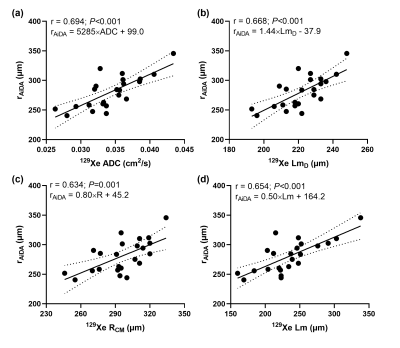
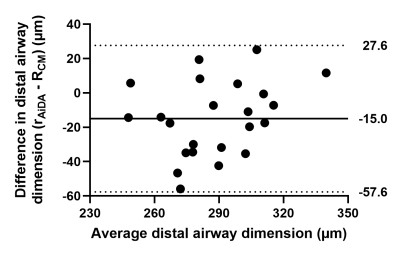
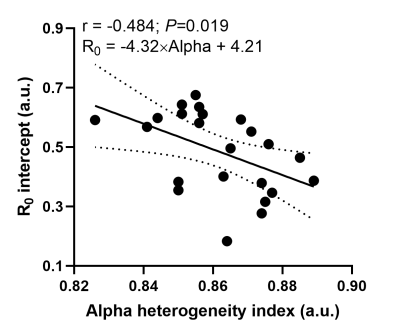
Table 1 summarizes volunteer demographics and mean global measurements from 129Xe DW-MRI and AiDA for this healthy cohort. 129Xe ADC and acinar airway dimensions lie between reported values for younger (29±4 years) healthy volunteers and ex-smokers3, demonstrating the prevalence of age-related acinar airway changes in this cohort of older healthy volunteers. This was supported by significant correlations (P<0.05) between volunteer age and all 129Xe DW-MRI metrics, except for alpha heterogeneity index. AiDA derived rAiDA and R0 intercept measurements in this healthy cohort were comparable to previously reported values in a similarly aged volunteers6. A trend towards increased rAiDA with age was observed; however, this was not statistically significant (P=0.072).Statistically significant linear correlations were observed between rAiDA and all 129Xe DW-MRI metrics (P=0.001), except for alpha index, indicating that rAiDA is a measure of acinar airway dimensions (Figure 2). The 129Xe acinar airway dimension that showed the best agreement with the rAiDA measurement appeared to be RCM. Agreement was confirmed with Bland-Altman analysis, where a mean bias of -15.0 µm (95% agreement limits of -57.6 to 27.6 µm) towards rAiDA was obtained (Figure 3). This agreement between RCM and rAiDA is likely related to the similar underlying cylindrical tube/airway geometry that underpins both the CM and AiDA models, respectively. The remaining bias between the measurements could be attributed to the multiple breath-hold acquisition and longer diffusion times of AiDA, in contrast to the single breath-hold acquisition and millisecond diffusion time of 129Xe DW-MRI. The R0 intercept from AiDA significantly correlated with 129Xe alpha heterogeneity index (P=0.019) (Figure 4). This correlation could be representative of heterogeneity in the distal/acinar airways that both metrics are hypothesized to measure2,4.
Conclusion
This work has benchmarked estimates of root mean square airway radius from inhaled nanoparticles (rAiDA) against 129Xe DW-MRI metrics in a healthy volunteer cohort, and the significant correlations indicate that rAiDA is a measure of acinar airway microstructure. Further benchmarking in non-symptomatic smokers could be used to evaluate the relative sensitivity of AiDA and 129Xe DW-MRI in detecting early emphysematous changes to the acinar microstructure.Acknowledgements
This work was supported by NIHR grant NIHR-RP-R3-12-027 and MRC grant MR/M008894/1. The views expressed in this work are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.References
1. Yablonskiy DA, Sukstanskii AL, Woods JC, et al. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol (1985). 2009;107(4):1258-65.
2. Chan HF, Stewart NJ, Norquay G, et al. 3D diffusion-weighted (129) Xe MRI for whole lung morphometry. Magn Reson Med. 2018;79(6):2986-95.
3. Chan HF, Collier GJ, Weatherley ND, et al. Comparison of in vivo lung morphometry models from 3D multiple b-value (3) He and (129) Xe diffusion-weighted MRI. Magn Reson Med. 2019;81(5):2959-71.
4. Londahl J, Jakobsson JK, Broday DM, et al. Do nanoparticles provide a new opportunity for diagnosis of distal airspace disease? Int J Nanomedicine. 2017;12:41-51.
5. Jakobsson JK, Hedlund J, Kumlin J, et al. A new method for measuring lung deposition efficiency of airborne nanoparticles in a single breath. Sci Rep. 2016;6:36147.
6. Aaltonen HL, Kindvall SS, Jakobsson JK, et al. Airspace Dimension Assessment with nanoparticles reflects lung density as quantified by MRI. Int J Nanomedicine. 2018;13:2989-95.
Figures