0428
Feasibility of Structural and Phase-Resolved Functional Lung (PREFUL) MRI in Free-Breathing Neonates1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3MR Predevelopment, Siemens Healthcare, Erlangen, Germany, 4Diagnostic and Intervetional Radiology, Hannover Medical School, Hannover, Germany, 5Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Member of the German Center for Lung Research (DZL), Hannover, Germany, 6MR Collaborations North East, Siemens Healthineers, Boston, MA, United States, 7Diagnostic Imaging, The Hospital for Sick Children, Toronto, ON, Canada, 8Medical Imaging, University of Toronto, Toronto, ON, Canada, 9Neurosciences and Mental Health, The Hospital for Sick Children, Toronto, ON, Canada, 10Neurology, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
MRI of the neonatal pulmonary system can be a useful tool for the clinical evaluation of lung structure and function without ionizing radiation. Despite this, the inherent challenges associated with MRI of the lung make this difficult. This works demonstrates the feasibility of structural and functional imaging of the neonatal lung without exogenous contrast using a clinical whole-body 3T system with standard coils in neonates without any cardiorespiratory history. The imaging protocol includes T1-weighted, T2-weighted, and ultrashort echo time (UTE) imaging for structural imaging as well as novel free-breathing Phase-Resolved Function Lung (PREFUL) MRI for ventilation/perfusion imaging.
Introduction
Structural and functional imaging of the neonatal pulmonary system may play a role in the clinical assessment of disease, especially in the case of diseases of prematurity such as bronchopulmonary dysplasia (BPD)1,2. Unfortunately most widely adopted clinical imaging techniques (chest radiograph and computed tomography) use ionizing radiation, making them less desirable for longitudinal assessment due to radiosensitivity of the neonatal lung. MRI is potentially more suitable, but is hampered by several limitations including the low 1H density of lung tissue, short T2*, and artifacts due to cardiorespiratory motion. However in recent years, improvements in image acquisition and reconstruction have begun to overcome these limitations and MRI has shown promise in the evaluation of neonatal BPD3. For example, ultrashort echo time (UTE) imaging allows for increased signal from the pulmonary parenchyma permitting structural evaluation of neonatal lungs4. There is also interest in functional lung imaging with dynamic, free-breathing acquisitions which exploit cardiorespiratory motion to generate ventilation and perfusion-weighted images without requiring an inhaled tracer gas (i.e. hyperpolarized gas) or intravenous contrast agent (i.e. gadolinium). These methods, such as Phase-Resolved Functional Lung (PREFUL)5 MRI are performed during relaxed tidal breathing, typically using standard gradient echo (GRE) sequences making them more amenable to widespread implementation, particularly in children. To our knowledge, PREFUL has not previously been demonstrated in neonates. The purpose of this work was to demonstrate the feasibility of performing a comprehensive neonatal pulmonary MRI study involving structural UTE and functional PREFUL MRI, alongside other conventional structural imaging. Healthy gross pulmonary perfusion was also evaluated using advanced high-resolution 4D-flow MRI.Methods
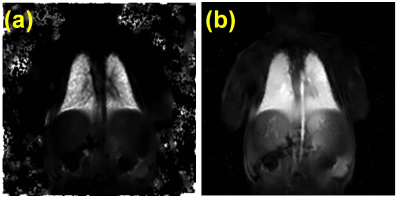
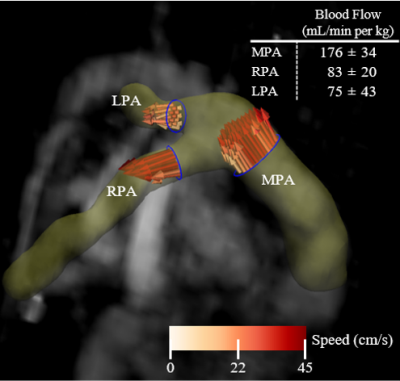
Eight term neonates with no history of cardiorespiratory issues were imaged with consent in accordance with ethics approval at the Hospital for Sick Children. Two were fed and swaddled for imaging at 5 and 19 weeks of age, while the other six were imaged within the first week of life, some with sedation. Imaging was performed on a clinical 3T system (Magnetom Prisma, Siemens Healthcare, Erlangen, Germany) with standard spine/thoracic coils. Imaging parameters are found in Table 1. UTE imaging was performed with a prototype stack-of-spirals 3D-VIBE sequence6 during quiet breathing and expiratory images were retrospectively reconstructed. Structural images of the lung including UTE, as well as T1- and T2-weighted images, were scored by a fellowship-trained pediatric radiologist (19 years experience reading MRI) according to the modified Ochiai scheme with a range of 0-143,4. Functional imaging was performed using conventional dynamic 2D-GRE (512 images) and processed using a PREFUL analysis software prototype (MRLung, Siemens Healthcare, Erlangen, Germany)5. Fractional Ventilation (FV) maps were extracted, normalized to their highest value, and ventilation defect percent (VDP) was calculated7,8. Similarly, perfusion maximum intensity projections (MIPs) were extracted, normalized, and perfusion defect percent (QDP) was calculated9, with thresholding similar to Kirby et al7. Finally, motion-robust respiratory-resolved 4D-flow was performed in a subset of five neonates to assess macrovascular blood supply to the lungs10. Blood flow was measured at end-expiration in major pulmonary arteries. The total MRI protocol duration was approximately 20-25 minutes.Results
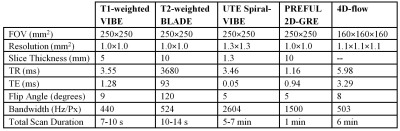
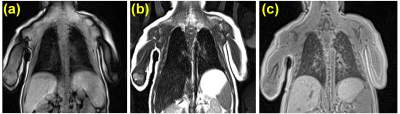
Representative T1-weighted, T2-weighted, and UTE images are shown in Figure 1. All lung images were observed to be of reasonably good quality with few artifacts in the thoracic cavity. Across all patients, the Ochiai scores were 1.1±1.3. Representative FV and perfusion MIP images calculated with PREFUL are shown in Figure 2. PREFUL ventilation maps were homogeneous with mean image-wide FV across all subjects of 0.59±0.07 and low VDP 0.80±0.76%, indicative of healthy ventilation (Figure 3). Similarly, PREFUL perfusion maps were homogeneous with mean image-wide QDP of 0.42±0.49% (Figure 3). Pulmonary flow values measured by 4D-flow (Figure 4) were consistent with those previously reported in healthy neonates using ultrasound11, indicating no major abnormalities.Discussion
This study demonstrates the feasibility of performing structural and functional lung imaging in free-breathing neonates using a clinical whole-body 3T scanner with standard coils. As expected for this population with no history of pulmonary disease, the structural Ochiai scores reported by a trained observer were low and comparable to scores reported previously in control term gestation neonates4. PREFUL ventilation and perfusion maps were homogenous, and measures of VDP and QDP were low, indicating normal lung function. This is supported by recent works showing agreement between PREFUL VDP and QDP with other measures of lung ventilation and perfusion (i.e. hyperpolarized 129Xe12 and gadolinium-enhanced MRI9, respectively). However, due to scan time constraints, the PREFUL analysis shown here was limited to a single 2D slice which may not fully capture the extent of underlying pathology. This limitation may be addressed in the future using volumetric PREFUL analysis13,14. Nevertheless, PREFUL MRI holds significant potential for functional lung imaging because of the low barrier of implementation making it more readily adaptable for clinical evaluation in neonates.Conclusion
Structural and functional imaging of the lung is feasible in healthy neonates using a clinical MRI system with and without sedation. The techniques described here hold promise for the evaluation of neonatal diseases such as diseases of prematurity (e.g. BPD), congenital heart and lung defects, as well as longitudinal monitoring without the need for exogenous contrast.Acknowledgements
The authors acknowledge the contributions of Saidah Hack, Daphne Kamino, Ashley LeBlanc, Daniel Li, Leslie Burns, Tammy Rayner, and Ruth Weiss for data collection. The authors thank the following sources of funding: Siemens Healthineers, the Ontario Research Fund (ORF), and Natural Sciences and Engineering Research Council (NSERC) of Canada.References
1. T. Semple, M.R. Akhtar, and C.M. Owens, “Imaging Bronchopulmonary Dysplasia — A Multimodality Update,” Front. Med. 4(88), 1–7 (2017).
2. L. Duijts, E.R. van Meel, L. Moschino, et al., “European Respiratory Society guideline on long term management of children with bronchopulmonary dysplasia,” Eur. Respir. J. In press (2019).
3. L.L. Walkup, J.A. Tkach, N.S. Higano, et al., “Quantitative Magnetic Resonance Imaging of Bronchopulmonary Dysplasia in the Neonatal Intensive Care Unit Environment,” Am. J. Respir. Crit. Care Med. 192(10), 1215–1222 (2015).
4. N.S. Higano, D.R. Spielberg, R.J. Fleck, et al., “Neonatal pulmonary magnetic resonance imaging of bronchopulmonary dysplasia predicts short-term clinical outcomes,” Am. J. Respir. Crit. Care Med. 198(10), 1302–1311 (2018).
5. A. Voskrebenzev, M. Gutberlet, F. Klimeš, et al., “Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients,” Magn. Reson. Med. 79(4), 2306–2314 (2018).
6. J.P. Mugler III, C.H. Meyer, J. Pfeuffer, A. Stemmer, and B. Kiefer, “Accelerated Stack-of-Spirals Breath-hold UTE Lung Imaging,” Proc. Intl. Soc. Mag. Reson. Med. 25, 4904 (2017).
7. M. Kirby, M. Heydarian, S. Svenningsen, et al., “Hyperpolarized 3He Magnetic Resonance Functional Imaging Semiautomated Segmentation,” Acad. Radiol. 19(2), 141–152 (2012).
8. G. Santyr, N. Kanhere, F. Morgado, J.H. Rayment, F. Ratjen, and M.J. Couch, “Hyperpolarized Gas Magnetic Resonance Imaging of Pediatric Cystic Fibrosis Lung Disease,” Acad. Radiol. 26(3), 344–354 (2019).
9 T.F. Kaireit, A. Voskrebenzev, M. Gutberlet, et al., “Comparison of quantitative regional perfusion-weighted phase resolved functional lung (PREFUL) MRI with dynamic gadolinium-enhanced regional pulmonary perfusion MRI in COPD patients,” J. Magn. Reson. Imaging 49(4), 1122–1132 (2019).
10. E.M. Schrauben, J.M. Lim, D.S. Goolaub, D. Marini, M. Seed, and C.K. Macgowan, “Motion robust respiratory-resolved 3D radial flow MRI and its application in neonatal congenital heart disease,” Magn. Reson. Med. (2019) [Published online ahead of print] 10.1002/mrm.27945.
11. W.P. de Boode, R. van der Lee, B.H. Eriksen, et al., “The role of Neonatologist Performed Echocardiography in the assessment and management of neonatal shock,” Pediatr. Res. 84(1), 57–67 (2018).
12. M.J. Couch, J.H. Rayment, R. Grimm, et al., “Comparison of Phase-Resolved Functional Lung (PREFUL) MRI and Hyperpolarized 129Xe MRI in Pediatric Cystic Fibrosis,” Proc. Intl. Soc. Mag. Reson. Med. 27, 0006 (2019).
13. A. Voskrebenzev, T.F. Kaireit, M. Gutberlet, et al., “Real-Time Imaging during Free-Breathing for Patient-Friendly V/Q Scan of the Whole Lung in One Minute at 3T,” Proc. Intl. Soc. Mag. Reson. Med. 27, 4081 (2019).
14. F. Klimeš, A. Voskrebenzev, M. Gutberlet, et al., “Feasibility of 3D PREFUL: 3D dynamic lung ventilation imaging, initial comparison to 2D PREFUL in healthy volunteers,” Proc. Intl. Soc. Mag. Reson. Med. 27, 1896 (2019).
Figures