0424
IDH1 genotype prediction in lower-grade gliomas: a machine learning study with VASARI and ADC radiomics
Shiteng Suo1, Mengqiu Cao1, Xiaoqing Wang1, Wei Yang2, Jianrong Xu1, and Yan Zhou1
1Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2Guangdong Provincial Key Laboratory of Medical Image Processing, School of Biomedical Engineering, Southern Medical University, Guangzhou, China
1Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2Guangdong Provincial Key Laboratory of Medical Image Processing, School of Biomedical Engineering, Southern Medical University, Guangzhou, China
Synopsis
Preoperative noninvasive prediction of IDH mutation status is crucial for prognosis and therapeutic decision making. In this study, we evaluated the qualitative and quantitative MRI features, namely, Visually Accessible Rembrandt Images (VASARI) features and apparent diffusion coefficient radiomics features in identifying IDH1 mutation status in lower-grade gliomas (WHO grade II-III). Results by machine learning methods showed that the combination achieved a better prediction performance. Our model may have the potential to serve as an alternative to the conventional workflow for the noninvasive identification of the molecular profiles.
INTRODUCTION
Isocitrate dehydrogenase (IDH) is one of the most important molecular biomarkers in gliomagenesis. Preoperative prediction of IDH mutation status is crucial for prognosis and therapeutic decision making. MRI can facilitate better preoperative diagnosis noninvasively. This study aimed to develop a machine learning approach based on qualitative Visually Accessible Rembrandt Images (VASARI) and quantitative ADC radiomics features and to examine its predictive value to identify the IDH1 mutation status in lower-grade gliomas (LGGs).METHODS
Local institutional review board approved the study with a waiver of the written informed consent from patients. A total of 102 LGG patients (60 men and 42 women; age range, 18-77 years; mean age, 45.3 ± 16.3 years) were included. Subjects were randomly divided into two subsets, a training cohort (n = 67) and a validation cohort (n = 35). All MRI scans were acquired on a 3 Tesla MRI system (Signa HDxt; GE Medical System) with an eight-channel head coil. The protocol included native T1W, T2W, FLAIR, and DWI in the axial plane and post-contrast T1W in three orthogonal planes. DWI was performed with b values of 0 and 1000 sec/mm2. Each tumor was scored according to the VASARI lexicon, which consists of 23 imaging traits related to the morphology of brain tumors. For quantitative ADC analysis, segmentation of the tumor area was first manually performed using 3D Slicer software. A total of 56 radiomics features were then extracted from the volumetric ADC data including shape, histogram and high-order texture features using Matlab software (version 2016a). Feature selection was conducted using the maximum Relevance Minimum Redundancy method and 0.632 + bootstrap method. A machine-learning model to predict IDH1 mutation status was established using the selected features and a random forest classifier. Predictive models of different orders (1–5 for VASARI features and 1–10 for radiomics features) were constructed separately on the optimal combinations of VASARI and radiomics features. Further, the fusion model from the optimal VASARI model and radiomics model was obtained by integrating the predicted probability of both models. Weighted average fusion rule was adopted for classifier fusion. The predictive performance was evaluated using receiver operating characteristic (ROC) curves. Area under the ROC curve (AUC), sensitivity, specificity, and accuracy were calculated.RESULTS
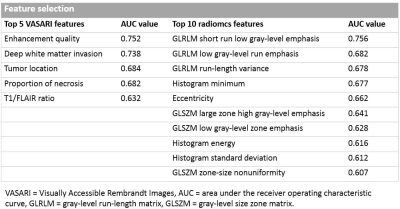
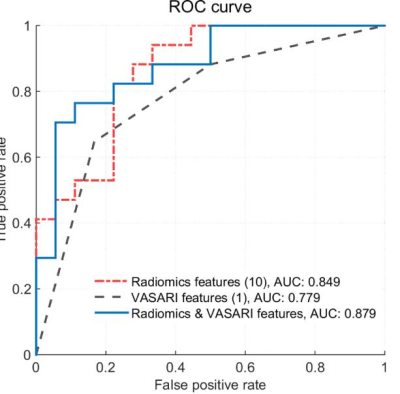
After feature selection, the top 5 VASARI features were enhancement quality, deep white matter invasion, tumor location, proportion of necrosis, and T1/FLAIR ratio. Prediction models with orders 1 to 5 were generated by incorporating the above optimal features. On the training cohort, the highest AUC of 0.827±0.031 was reached, with a sensitivity of 0.671±0.058 and a specificity of 0.712±0.049, respectively. Using the optimal feature set (the single enhancement quality feature), the trained model achieved an AUC of 0.779±0.001 on the validation cohort, with a sensitivity of 0.718±0.070, a specificity of 0.733±0.100, and an accuracy of 0.726±0.017, respectively.In ADC radiomics analysis, the top 10 quantitative features were listed in Table 2. On the training cohort, the highest AUC of 0.849±0.027 was reached, with a sensitivity of 0.790±0.038 and a specificity of 0.770±0.043, respectively. Using the optimal feature set (all the 10 features), the trained model achieved an AUC of 0.849±0.008 on the validation cohort, with a sensitivity of 0.724±0.035, a specificity of 0.761±0.017, and an accuracy of 0.743±0.022, respectively.
The fusion model of the optimal VASARI model (enhancement quality) and radiomics model (the top 10 radiomics features) improved the AUC to 0.879, with a sensitivity of 0.765, a specificity of 0.778, and an accuracy of 0.771, respectively.