0416
Whole-tumor radiomics analysis of DKI and DTI may improve the prediction of genotypes for astrocytomas: a preliminary study1Department of Radiology, First Hospital of Shanxi Medical University, Taiyuan, China, 2College of Medical Imaging, Shanxi Medical University, Taiyuan, China, 3Department of Cancer Physiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States
Synopsis
Assessment of glioma genotypes by quantifying MR diffusion imaging heterogeneity of whole tumour may serve as a powerful tool to instruct therapeutic decision-making. This study evaluated the role and incremental value of whole-tumor radiomics analysis based on DKI and DTI images in determining the IDH and MGMTmet genotypes of astrocytomas. A radiomics models based on whole-tumor MK and MD maps showed good diagnostic efficiency in predicting IDH and MGMTmet genotypes. Furthermore, the combined model constructed by radiomics score, edema degree and age further improved the performance of predicting IDH, while the combined model did not benefit for MGMTmet prediction.
Background and Purpose
Diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI) can probe the pathological changes in gliomas, providing abundant important information.1 Radiomics allow for more precise diagnosis, prediction of survival, and assessment of therapeutic response in glioma than traditional imaging biomarkers, which can thus offer a complementary tool to existing radiological practice.2-4 Tumors are heterogeneous both genetically and histopathologically, with intratumoural spatial variation in the cellularity, angiogenesis, areas of necrosis and extravascular extra-cellular matrix, which might result in the diffusion heterogeneity of whole tumour.5 Thus, we postulated that the heterogeneity of DKI and DTI parameter values within whole gliomas could be useful for predicting IDH and MGMTmet genotypes. Therefore, this study evaluated the role and incremental value of whole-tumor radiomics analysis based on DKI and DTI images in determining the IDH and MGMTmet genotypes of astrocytomas.Method
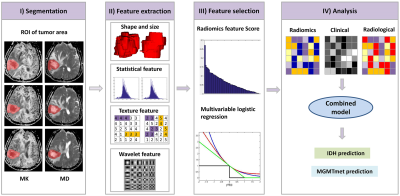
Sixty-two patients with astrocytoma (33 males and 29 females) were enrolled. Tumors were graded using the World Health Organization Classification of Tumors of the Nervous System (2016) criteria. The clinical characteristics of gender, age and grade were also studied. IDH status of the patients was determined using Sanger sequencing, the MGMTmet was determined by pyrosequencing analysis. Preoperative MRI was performed with a 3.0-T scanner using an 8-channel array coil. The scanning sequences included conventional MRI sequences, DKI and DTI sequences. Echo planar imaging (EPI) sequence was used to perform DTI and DKI. Implemented b values were 0, 1000, and 2000 mm2/s for DKI (including DTI). These were applied in 30 uniformly distributed directions. Parameters for DKI data: TR/TE: 6500/11 ms; FOV, 240 mm × 240 mm; matrix, 96 × 96. Slice thickness, 6 mm; slice interval, 1 mm. Quantitative semantic radiological characteristics were assessed, which included tumour size, border, hemorrhage, cystic and necrosis, edema degree, enhancement style and degree, signal characteristics, tumour location, cross midline growth, involving deep white matter, involving pia mater, involving ependymal membrane. 364 radiomics features of whole tumor were extracted from mean-kurtosis (MK), and mean-diffusivity (MD) images, respectively (Figure 1). The ROIs segmentation of the MRI images is showed in Figure 2. The multivariable logistic regression was used to select the most meaningful radiomics features for predicting IDH and MGMTmet genotypes. A radiomics model was built by logistic linear regression. A combined model was established based on selected radiomic, radiological and clinical features. To assess the difference between the models, the Z-test was performed.Result
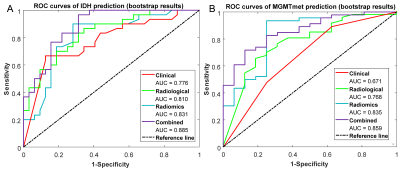
For IDH prediction, the univariate analysis revealed that significant differences existed in age (P = 0.001), grade (P = 0.004), edema degree (P < 0.001), and enhancement degree (P = 0.002) between IDHMUT and IDHWT groups. By further multivariable logistic regression analysis, the age and grade were selected to develop clinical model with AUC = 0.775, the MK value and edema degree were selected to develop radiological model with AUC = 0.810 (Figure 3A). For MGMTmet prediction, the univariate analysis showed that significant differences existed in grade (P = 0.026), edema degree (P < 0.001), border (P = 0.047), and enhancement degree (P = 0.035) between the MGMTmet and no MGMTmet groups. The clinical model was built by grade (AUC = 0.671), the radiological model was built by edema degree (AUC = 0.768) by further multivariate analysis (Figure 3B). The radiomics model built using the three most informative radiomics features for each genotype yielded an AUC of 0.831 ((95% confidence interval [CI]: 0.721-0.918) for predicting IDH genotype, and 0.835 (95%CI: 0.686-0.951) for MGMTmet genotype. A combined model for predicting IDH based on the radiomics score, age, and degree of edema reached an AUC of 0.885 (95%CI: 0.802-0.955) and a combined model for predicting MGMTmet based on radiomics score and edema degree reached an AUC of 0.859 (95%CI: 0.751-0.945) which was not significantly higher than the radiomics only model (P = 0.081) .Discussion and Conclusion
A radiomics models based on whole-tumor MK and MD maps showed good diagnostic efficiency in predicting IDH and MGMTmet genotypes of astrocytomas. Furthermore, the combined model constructed by radiomics score, edema degree and age further improved the performance of predicting IDH, while the combined model constructed by radiomics score and edema degree did not benefit the predictive performance of MGMTmet.Acknowledgements
This study was supported by the National Natural Science Foundation (81471652, 81771824 and 81971593 to Hui Zhang; 81701681 to Yan Tan; 81971592 to Xiao-chun Wang; 11705112 to Guo-qiang Yang); the Precision Medicine Key Innovation Team Project (YT1601 to Hui Zhang); the Social Development Projects of Key R&D Program in Shanxi Province (201703D321016 to Hui Zhang); the Youth Innovation Fund (YC1426 to Yan Tan); and the US National Cancer Institute, U01 CA143062 and U01CA200464 (Robert Gillies and Wei Mu).References
1. Raab P, Hattingen E, Franz K, et al., Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254(3):876-81.
2. Park JE, Kim HS, Radiomics as a quantitative imaging biomarker: practical considerations and the current standpoint in neuro-oncologic studies. Nuclear Medicine and Molecular Imaging. 2018;52:99-108.
3. Lao J, Chen Y, Li ZC, et al., A deep learning-based radiomics model for prediction of survival in glioblastoma multiforme. Sci Rep. 2017;7(1):10353.
4. Gillies RJ, Kinahan PE, Hricak H, Radiomics: Images are more than Pictures, they are Data. Radiology. 2016;278:563-577.
5. Kleihues P, Soylemezoglu F, Schauble B, et al., Histopathology, classification, and grading of gliomas, Glia. 1995;15(3):211-221.
Figures