0411
Quantification of meningioma-brain adhesion using MR-elastography based slip interface imaging1Radiology, Mayo Clinic, Rochester, MN, United States, 2Neurosurgery, Mayo Clinic, Rochester, MN, United States, 3Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States
Synopsis
Brain tumor adherence has been long recognized to impact surgical resection difficulty. Recently-developed slip interface imaging (SII) can preoperatively predict tumor-brain adhesion. In previous studies, subjectively-determined SII assessment of tumor adhesion has been shown to agree well with intraoperative findings. The purpose of this work was to develop an objective quantitative method for analyzing SII data for adherence, thereby minimizing inter- and intraobserver variability. We developed a radiomics-based metric (termed “adhesion degree”) based on SII to quantify the degree of tumor adhesion. In 46 meningiomas, the adhesion degree showed excellent accuracy in predicting completely adherent tumors (AUROC=0.96) from non-adherent tumors.
Introduction
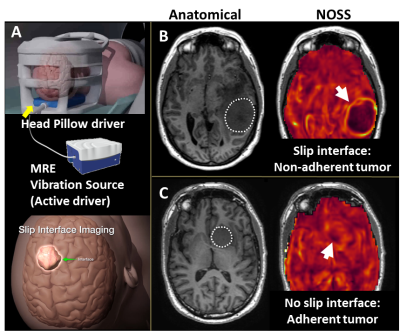
Adhesion of meningiomas to adjacent normal tissue has been long recognized to impact surgical resection difficulty and rate of surgical complications.1 Recently-developed slip interface imaging (SII) can preoperatively predict tumor-brain adhesion, assisting clinicians to more accurately assess the surgical risk and optimize the management plans.2,3 SII utilizes the principles of MR elastography (MRE) to detect slip interfaces as high normalized octahedral shear strain (NOSS) values resulting from the wave discontinuity across the tumor-brain boundary under an applied shear force (Fig.1). In previous studies, subjectively-assessed SII assessment of tumor adhesion has been shown to agree well with intraoperative assessment. The purpose of this work was to develop an objective quantitative method for analyzing SII data for adherence, thereby minimizing inter- and intraobserver variability. We developed a radiomics-based metric (termed “adhesion degree”) based on NOSS patterns in the tumor periphery to quantify the degree of tumor adhesion. The goal of this study was to determine the ability of this quantitative analysis of SII to assess tumor adhesion in patients with meningiomas.Methods
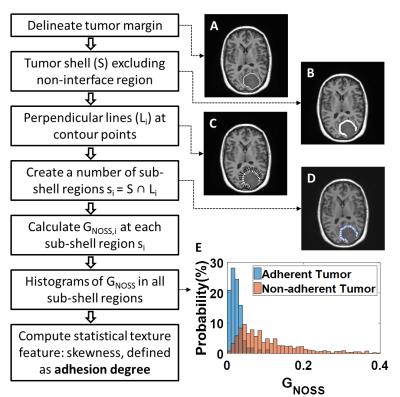
Data acquisition: With IRB approval and written informed consent, preoperative imaging was performed on 46 meningiomas in 45 patients (one patient had two meningiomas, 59.7±10.7years, 34F/11M) on 3T MR scanners. The imaging protocol included a single-shot, flow-compensated, SE-EPI-MRE and high-resolution anatomic T1W imaging.4Adhesion degree: NOSS maps were calculated from the measured displacement fields as previously described,3 and a NOSS shell feature was extracted (Fig.2). First, tumor margins were manually delineated from T1W images registered to the MRE space. With morphological operations, a tumor shell was formed consisting of 1 outer and 1 inner voxel around the tumor-brain interface while excluding non-interface regions. A perpendicular line was placed at each contour point, and a number of sub-shell regions were produced as the intersection of the tumor shell and each perpendicular line. The maximum gradient magnitude of the NOSS (GNOSS) at each sub-shell region was calculated. A non-adherent region with a visible NOSS contour is expected to have a large GNOSS. Finally, the histogram of GNOSS was constructed by quantizing the GNOSS of all sub-shell regions into 40 bins. The probability histogram of GNOSS (Fig.2E) represents the NOSS variation of voxels in the entire tumor-brain interface, indicating the adhesion of the tumor. As illustrated in Figure 2E, non-adhrent tumors have higher mean values and a broader distribution. However, the skewness turned out to be the best discriminator of adhesion, so the adhesion degree was defined as the skewness of the probability distribution of GNOSS.
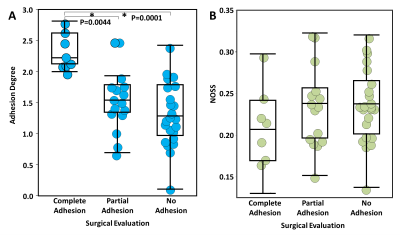
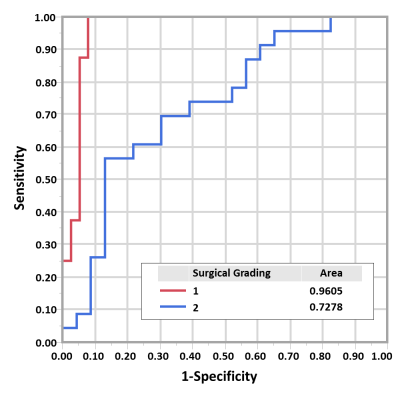
Statistical analyses: The tumors were grouped into 3 categories (0:complete adhesion, 1:partial, and 2:no adhesion) according to intraoperative adhesion assessment as previously described.3 A one-way ANOVA with post hoc multiple comparisons was performed to assess the group differences in adhesion degree (p<0.05). For comparison, mean NOSS values calculated from the tumor margin were also analyzed. Finally, the predictive accuracy of the adhesion degree was evaluated by ROC analyses.
Results
Figure 2E shows representative probability histograms of one adherent and one non-adherent meningioma, where the non-adherent tumor gives a lower GNOSS skewness (i.e., adhesion degree) than the adherent tumor, reflecting the differences in NOSS patterns in the tumor periphery. As shown in figure 3A, the adhesion degree of the completely adherent tumors were significantly higher than the partially and non-adherent tumors, while no significant difference was found between the partially and non-adherent tumors. In contrast, there was no significant difference in the mean NOSS among three groups (Fig.3B). The adhesion degree showed the highest accuracy in predicting complete adhesion (Fig.4, AUROC=0.96).Discussion
SII assesses the tumor adhesion using the contrast of NOSS at the tumor boundary. However, assessment of the existence of the hyper-intensity NOSS contour is performed by a radiologist’s qualitative evaluation, relying on relative contrast within the image, which is prone to inter- and intraobserver variability. Moreover, as shown in figure 3B, the absolute values of NOSS also vary from scan to scan due to differences in the head-driver mechanical coupling for each patient, which hinders its use in SII quantification. In this study, we constructed a radiomics-based metric (adhesion degree) from the periphery pixels around the tumor NOSS edge to quantify the degree of tumor adhesion. The strength of this metric is the use of a simple description that captures the NOSS patterns across the tumor boundary. Our results show the adhesion degree can successfully distinguish completely adherent tumors, which require tedious dissection away from surrounding tissues, from non-adherent tumors. However, its ability in distinguishing between partial and no adhesion is limited. This may due to the qualitative nature of our reference standard, where surgical excision of brain tumors is performed piece-by-piece in a small visual field, making it difficult at times to accurately quantify the percentage of tumor adhesion. Future work with our neurosurgeons will establish a more precise scale by incorporating precise spatial localization using image-guided stereotactic resection data. Another limitation of this technique is the manual tumor segmentation that is time-consuming and subjective. Automatic tumor segmentation is expected to improve segmentation accuracy.Conclusion
The SII-derived adhesion degree provides a quantitative assessment and increased sensitivity of meningioma adhesion compared with traditional qualitative visual inspection of NOSS maps.Acknowledgements
This work was supported by grants from the NIH (R01 EB001981, RO1 EB010065, R01 NS113760) and Mayo Clinic imaging award CIM-92541587.References
1. van Alkemade H, de Leau M, Dieleman EM, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14:658–666.
2. Yin Z, Glaser KJ, Manduca A, Van Gompel JJ, Link JM, Hughes JD, Romano A, Ehman RL, Huston J. Slip Interface Imaging Predicts Tumor-Brain Adhesion in Vestibular Schwannomas. Radiology. 2015; 277: 507-517.
3. Yin, Z, Hughes JD, Trzasko JD, Glaser KJ, Manduca A, Van Gompel JJ, Link JM, Hughes JD, Romano A, Ehman RL, Huston J.. Slip interface imaging based on MR-elastography preoperatively predicts meningioma–brain adhesion. J Magn Reson Imaging. 2017;46: 1007-1016.
4. Murphy MC, Huston J, 3rd, Glaser KJ, et al. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J Neurosurg. 2013;118(3):643-8.
Figures