0410
Preoperative assessment of stiffness and tumor-brain adhesion in schwannoma and meningioma patients and its comparison with surgical findings1Radiology, Ohio State University Wexner Medical Center, Columbus, OH, United States, 2Otolaryngology, Ohio State University Wexner Medical Center, Columbus, OH, United States, 3Ohio State University Wexner Medical Center, Columbus, OH, United States, 4Neurological Surgery, Ohio State University Wexner Medical Center, Columbus, OH, United States
Synopsis
Microsurgery in brain tumor patients aim to complete tumor resection without compromising neurological functionality. Inadequate preoperative knowledge of tumor may prolong surgical time and increase risk of postoperative complications which may depends upon tumor-brain adhesion and tumor stiffness. Previous studies have only looked into adhesion and stiffness separately using Magnetic Resonance Elastography (MRE). Previously, we proposed both adhesion and stiffness in vestibular schwannoma patients. Aim of this study is to also include meningioma cases in the analysis in order to broaden the tumor types and complexity. Preliminary results show good correlation between MRE-derived preoperative assessment of tumor and surgical findings.
Purpose
For decades, patients with brain tumor such as vestibular schwannoma (VS) and meningioma have been treated with microsurgery to resect the tumor. Surgery involves peeling tumor off the brain as well as resecting, which depends upon tumor-brain adhesion and tumor stiffness, respectively. Inadequate preoperative knowledge of tumor stiffness and tumor-brain adhesion may prolong surgical time and increase risk of postoperative complications. A study by Sakai et al. has shown stiffness estimates using magnetic resonance elastography (MRE) in patients with brain tumors including vestibular schwannoma have found a significant correlation with tumor consistency during surgery [1]. Study by Yin et al. has investigated tumor-brain adhesion in patients with vestibular schwannoma [2] and meningioma [3]. As per our knowledge, none of the earlier studies have investigated both the tumor stiffness and tumor-brain adhesion together in both meningioma and schwannoma brain tumor patients. Previously, we proposed schwannoma stiffness and tumor-adhesion using local frequency estimation (LFE) inversion technique, which has its limitations. The aim of this study is to compute tumor stiffness using direct inversion (DI) method and tumor-brain adhesion in patients with vestibular schwannoma and meningioma and correlate with the clinical assessment score reported by surgeons during surgery.Methods
All imaging was performed using a 3T MRI scanner (Tim Trio and Prisma, Siemens Healthcare, Erlangen, Germany). Written informed consent was obtained from all patients combining schwannomas and meningiomas (n=22; age range: 26-69years). Axial slices were obtained using a spin-echo echo planar imaging (SE-EPI) MRE sequence. T2-weighted and T1-weighted fluid-attenuated inversion recovery (FLAIR) images were acquired to clearly identify the tumor-brain interface. 60 Hz vibrations were introduced through a soft pillow-like driver that is placed underneath head in the brain coil. Imaging parameters included: FOV=256x256mm2, matrix size=256x256, TR/TE: 1800/43.4ms, slice thickness=3 mm, ~16 slice based on tumor coverage, MRE phase offsets=4. Motion encoding gradient of 60 Hz was applied separately in the x, y and z directions to encode in-plane and through plane displacement fields. Total scan time was ~ 6 minutes. MRE images were masked to obtain the brain and a curl processing was performed to remove longitudinal component of motion. Additionally, directional filter was applied to remove the reflected waves. Finally, DI method with laplacian of Gaussian was performed to compute weighted stiffness map. ROI was drawn couple of pixels away from the brain boundary to avoid any edge effects. On MRE data, octahedral shear strain (OSS) [4] algorithm was applied to determine shear strain measurements at the tumor-brain interface to quantify the degree of adhesion. OSS map from each patient was then normalized by dividing the OSS map by its median value since the vibrations reaching the tumor-brain interface may vary in amplitude from patient to patient depending on anatomy, tumor location and contact with the soft driver. Mean value from five randomly selected pixels on tumor brain interface from brainstem (BS) and cerebellum (CER) regions is reported for OSS. Finally, minimum value of the two regions, BS and CER, is reported.Blinded to the preoperative results, neurosurgeons qualitatively assessed tumor consistency (scale 1 to 5) and adherence (scale 0 to 5) for BS and CER regions during the surgery. Only 8 patients out of 22 have surgical scores by surgeons for adherence and 7 out of 22 had surgical consistency results.
Results
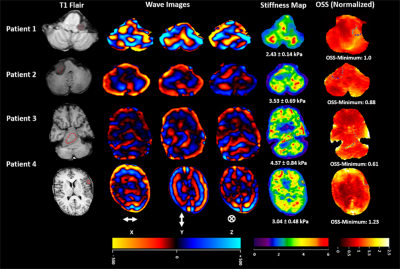
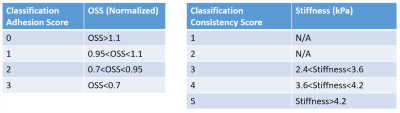
Table 1 illustrates the scoring scale used to report surgical finding by surgeons.Figure 1 shows T1 flair localizer, wave images in x, y and z directions, MRE-derived stiffness map and OSS normalized map. Patient 1 and 2 have schwannoma and patient 3 and 4 have meningioma.
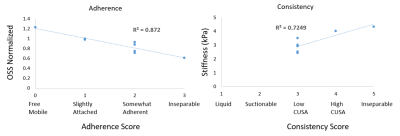
Figure 2 shows linear correlation plot between MRE predictions and surgical findings for adherence in 8 and consistency in 7 brain tumor patients. Good correlation was found in adherence with R2 = 0.87 and consistency with R2 = 0.72.
Table 2 shows classification generated based on patients data with surgical findings.
Conclusion
This study shows a good correlation between MRE predictions and surgical findings in patients with brain tumor such as schwannoma and meningioma.Acknowledgements
Funded by NIH R01HL124096.References
1. Sakai, N., et al. "Shear stiffness of 4 common intracranial tumors measured using MR elastography: comparison with intraoperative consistency grading." American Journal of Neuroradiology 37.10 (2016): 1851-1859.
2. Yin, Ziying, et al. "Slip interface imaging predicts tumor-brain adhesion in vestibular schwannomas." Radiology 277.2 (2015): 507-517.
3. Yin, Ziying, et al. "Slip interface imaging based on MR‐elastography preoperatively predicts meningioma–brain adhesion." Journal of Magnetic Resonance Imaging 46.4 (2017): 1007-1016.
4. McGarry, M. D. J., et al. "An octahedral shear strain-based measure of SNR for 3D MR elastography." Physics in Medicine & Biology 56.13 (2011): N153.
Figures