0406
The Effect of Tumor Grade within IDH Wild-Type and IDH Mutant Gliomas Assessed by Proton Magnetic Resonance Spectroscopy at 3T1Institute of Biomedical Engineering, Bogaziçi University, Istanbul, Turkey, 2Brain Tumor Research Group, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 3Department of Pathology, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 4Department of Molecular Biology and Genetics, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 5Center for Neuroradiological Applications and Research, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 6Department of Medical Device Technologies, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 7Department of Neurosurgery, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 8Department of Radiology, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey
Synopsis
Isocitrate dehydrogenase (IDH) mutation highly affects the overall survival of gliomas. In addition to the IDH mutation, the tumor histologic grade might play a role in patient prognosis. The aim of this study was to assess the metabolic variations between different tumor grades within IDH mutant (IDH-mut) and IDH wild-type (IDH-wt) gliomas using proton MR spectroscopy. Higher glycine in glioblastoma (GBM) within IDH-mut, and lower Cr and mIns in GBM within IDH-wt were the only statistically significant differences. Our study indicated similar metabolic profiles of different grades within IDH mutational subgroups, supporting IDH as a better predictor of clinical outcome.
Introduction
Recent studies have marked the importance of isocitrate dehydrogenase (IDH) mutation in overall survival of gliomas, and WHO classification of central nervous system tumors incorporated IDH mutation into the diagnosis of gliomas.1, 2 Brain tumors with IDH mutation (IDH-mut) have better prognosis and treatment response than IDH wild-type (IDH-wt) tumors.3-8 Proton magnetic resonance spectroscopy (1H-MRS) provides biomarkers of cellular metabolism, and previous 1H-MRS studies indicated that IDH-mut tumors displayed a less aggressive metabolic profile than other tumors based on higher 2-hydroxyglutarate (2HG)9-11, myo-inositol (mIns)12, and N-acetyl aspartate (NAA)12, and lower gluthathione (GSH)12, 13, glycine (Glyc)12, total choline (tCho)12, glutamate (Glu)12, 14, and glutamate glutamine complex (Glx)12 levels. On the other hand, tumor histologic grade has been the long-standing gold standard of assessing tumor behaviour, and the overall survival for low-grade gliomas (LGG) is better than glioblastoma (GBM).15 There has not been a report on MRS markers of different tumor grades within IDH-mut and IDH-wt gliomas. The aim of this study is to define MR spectroscopic differences of diffuse glioma groups classified by grade within IDH-mut and IDH-wt gliomas at 3T.Methods
A total of 112 patients diagnosed with a diffuse-glioma (70M/42F, mean age: 42.08±13.88 years, range: 20-74 years, 73 IDH-mut, 39 IDH-wt, 33 glioblastoma (GBM), 31 anaplastic astrocytoma, 9 grade III anaplastic oligodendroglioma, 21 grade II diffuse astrocytoma, and 18 grade II oligodendroglioma) were scanned before surgery at a Siemens Tim Trio clinical 3T scanner (Erlangen, Germany) using a 32-channel head coil. 1H-MRS data were acquired from the solid tumor region with the maximum cerebral blood volume (CBV) excluding gross hemorrhage, edema, and necrosis by using a short echo time (TE) Point Resolved Spectroscopy (PRESS) sequence (repetition time (TR)=2000 ms, TE=30ms, 1024 points, 1200 Hz, voxel size= 10x10x10 mm3, number of signal averages= 192, acquisition time=6.5 min). The spectral peak intensities for 19 metabolites, including creatine (Cr), phosphocreatine (PCr), gamma-aminobutyric acid (GABA), glutamine (Gln), Glu, Glyc, glycerophosphocholine (GPC), phosphocholine (PCh), GSH, 2HG, mIns, lactate (Lac), NAA, N-acetylaspartylglutamic acid (NAAG), alanine, aspartate, glucose, scyllo-inositol, and taurine, in addition to macromolecules and lipids were quantified for each spectrum using LCModel with a simulated basis set.16 Alanine, aspartate, glucose, scyllo-inositol, and taurine were not quantifiable in more than 30% of the patients, and were excluded from further analysis after quantification. Additionally, for the remaining metabolites, any metabolite of a given spectrum with a Cramer-Rao lower bound (CRLB) of more than 30 was excluded from further analysis. Immunohistochemistry was performed for IDH1 (Diovana, H09). Afterwards, minisequencing was performed for IDH1-R132G/S/C, IDH1-R132L/H/P, IDH2-R140Q/L, IDH2-R140W, IDH2-R172K/M, and IDH2-R172W. A Kruskal Wallis test followed by post hoc Tukey-Kramer test was used to identify statistically significant MR spectroscopic differences between different grades within IDH-mut and IDH-wt gliomas. Bonferroni multiple comparison correction was applied, and a P value of less than 0.003 was considered as statistically significant.Results
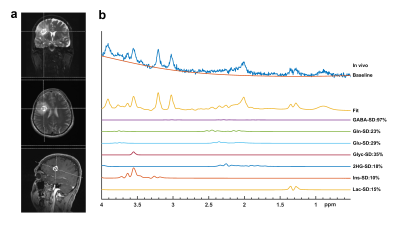
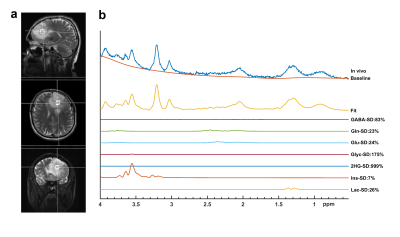
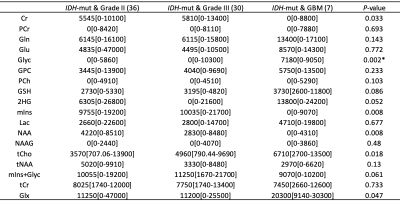
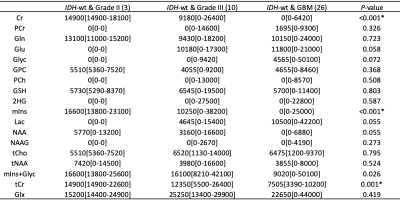
Figures 1 and 2 show example short-TE 1H-MRS data along with some of the LCModel results for an IDH-mut grade III oligodendroglioma and an IDH-wt GBM, respectively. While higher tCho, Lac and lipid peaks, and lower tNAA were observable in IDH-wt GBM, IDH-mut grade III oligodendroglioma displayed high 2HG and mIns. GBM patients had statistically significantly higher Glyc (P = 0.002) than grades II and III gliomas in IDH-mut group (Table 1). Additionally, GBM patients had statistically significantly lower Cr (P < 0.001), tCr (P = 0.001), and mIns (P < 0.001) than grades II and III in IDH-wt gliomas (Table 2). There were trends of lower Cr (P = 0.03), mIns (P = 0.008), and NAA (P = 0.008), and higher tCho (P =0.018) and Glx (P = 0.05) in GBM subgroup of IDH-mut gliomas. Moreover, IDH-wt GBM patients had a trend for lower mIns+Glyc (P = 0.026) than IDH-wt lower grade gliomas.Discussion and Conclusion
Since 2015, studies with long-term follow-up of large cohorts have shown distinct differences of clinical course between IDH-mut and IDH-wt tumors, and the malignancy definition is not based solely on histopathological findings anymore.17, 18 Tumors with the same morphology may have different molecular phenotypes 8, and molecular phenotype may be prognostically more relevant than morphology.19 Thus, World Health Organization (WHO) has included molecular features in the classification of gliomas in 2016.1 Diffuse glial tumors have completely different clinical behavior based on IDH and/or telomerase reverse transcriptase promoter (TERTp) mutation status. The morphologic criteria suggesting malignancy is also quite different for each molecular subtype. All IDH-wt glial tumors, even the ones with no mitosis or necrosis that would be grouped as grade II based on morphology, have worse prognosis than any IDH-mut astrocytic tumor, and these tumors are now considered as malignant. No statistically significant metabolic differences except glycine were found between different grades of IDH-mut gliomas, and lower Cr and mIns in GBM were the only statistically significant findings in IDH-wt group. In conclusion, the results of this study indicated that different grade gliomas had similar 1H-MRS profiles within IDH-mut or IDH-wt gliomas, which might be one of the reasons of similar clinical behavior despite the grade within IDH mutational subgroups.Acknowledgements
This research has been supported by TUBİTAK 1003 grant 216S432.References
1. Hoshide R and Jandial R. 2016 World Health Organization Classification of Central Nervous System Tumors: An Era of Molecular Biology. World Neurosurg. 2016; 94: 561-2.
2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131: 803-20.
3. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015; 372: 2499-508.
4. Killela PJ, Pirozzi CJ, Healy P, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014; 5: 1515-25.
5. Ogura R, Tsukamoto Y, Natsumeda M, et al. Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology. 2015; 35: 324-35.
6. Takano S, Ishikawa E, Sakamoto N, et al. Immunohistochemistry on IDH 1/2, ATRX, p53 and Ki-67 substitute molecular genetic testing and predict patient prognosis in grade III adult diffuse gliomas. Brain Tumor Pathol. 2016; 33: 107-16.
7. Yan H, Bigner DD, Velculescu V and Parsons DW. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res. 2009; 69: 9157-9.
8. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360: 765-73.
9. Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012; 4: 116ra4.
10. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012; 18: 624-9.
11. Elkhaled A, Jalbert LE, Phillips JJ, et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas.Sci Transl Med. 2012; 4: 116ra5.
12. Ozturk-Isik E, Cengiz S, Ozcan A, et al. Identification of IDH and TERTp mutation status using (1) H-MRS in 112 hemispheric diffuse gliomas. J Magn Reson Imaging. 2019.
13. Pope WB, Prins RM, Albert Thomas M, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012; 107: 197-205.
14. Nagashima H, Tanaka K, Sasayama T, et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro-Oncology 2016; 18: 1559-68.
15. Claus EB, Walsh KM, Wiencke JK, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. 2015; 38: E6.
16. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001; 14: 260-4.
17. Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta neuropathologica. 2015; 129: 867-73.
18. Hasselblatt M, Jaber M, Reuss D, et al. Diffuse Astrocytoma, IDH-Wildtype: A Dissolving Diagnosis. J Neuropathol Exp Neurol. 2018; 77: 422-5.
19. Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta neuropathologica. 2010; 120: 707-18.
Figures