0404
Glioma 2HG threshold setting based on normal appearing white matter increases the diagnostic value of 3D MEGA-LASER for IDH mutation detection1Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, United Kingdom, 2Institute of Neurology, University College London, London, United Kingdom, 3Department of Biomedical Engineering. School of Biomedical Engineering and Imaging Sciences, Kings College London, London, United Kingdom, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 5High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria
Synopsis
It has been established that 2-hydroxyglutarate (2GH) MRS is important for non-invasive diagnosis of isocitrate dehydrogenase (IDH) status that holds prognostic value for the patient and is important for treatment planning. The purpose of this work was to investigate whether the threshold determination for identification of IDH mutation could be improved by using ‘control’ spectra from normal-appearing white matter, NAWM , in multi-voxel 3D MEGA-LASER acquisitions. The proposed approaches to threshold determination for 2HG detection in the tumour voxels provided increased sensitivity and specificity as compared with the cut-off thresholds based on CRLB% relative error.
INTRODUCTION
The mutations in the isocitrate dehydrogenase (IDH) genes in human gliomas hold prognostic value for patients and are important for patient management 1-3. However this can only be reliably determined via histopathology. The abnormal accumulation of 2-hydroxyglutarate (2HG) which is upregulated in the mutant gliomas, can be detected non-invasively and robustly in 1H MR spectra by means of optimized acquisition techniques 4-6.However there is an open debate on whether a reliable threshold for 2HG detection can be established; the latter being essential for the clinical dissemination of the technique. It has recently been demonstrated that usage in standard practice of the accepted Cramer Rao Lower Bounds (CRLB)% is not suitable when dealing with low metabolite levels7.
The purpose of this work was to investigate whether the threshold determination for identification of IDH mutation could be substantially improved by using ‘control’ spectra from white matter appearing normal (normal-appearing white matter, NAWM) in the vicinity of tumours, in multi-voxel 3D MEGA-LASER8 acquisitions.
METHODS
Patients39 patients with suspected brain glioma consented to take part in this prospective study. Histopathology data became subsequently available, confirming IDH type, thus allowing retrospective comparison with MRS findings to validate the technique and its accuracy.
MR imaging, processing, analysis
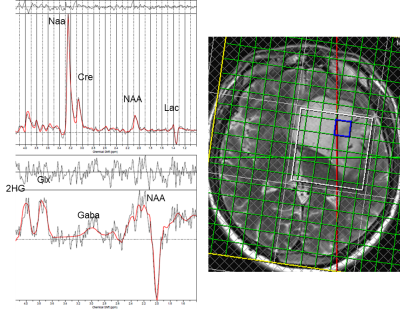
MR acquisitions were performed on a 3T Prisma scanner (Siemens, VE11C) using a 64-channel head coil. For MRS, 3D MEGA-LASER was used8. The sequence uses J-editing with the editing pulse set at 1.9 ppm, editing the 2HG resonance at 4.01 ppm. The multi-voxel approach can provide spatial information useful to characterize tumor heterogeneity. It can also provide control spectra from NAWM surrounding the tumour.
Acquisition parameters were: TR=1680ms, TE=68ms, FOV= 200x200x160mm3, acquisition matrix 10x10x8 resulting in 8ml voxels. The 3D volume was planned based on 2D T2-weighted TSE and 3D T2-weighted FLAIR images to cover as much of the lesion as possible whilst also including control voxels in NAWM.
MRSI data were evaluated using LCModel (v.6.3-1L) with simulated basis spectra containing 20 metabolites for non-edited spectra and 7 metabolites for edited spectra. Spectral quality was judged on the basis of reported SNR and linewidth; edited spectra with clear subtraction errors (e.g. visible Cr, Cho) were excluded.
2HG estimates were recorded without exclusion based on CRLB. 2HG concentrations are reported as relative ratios of 2HG to total Creatine (tCr, from non-edited spectra) and 2HG to NAA (present in edited spectra).
For 2HG detection/estimation, spectra from voxels located in the center of the tumor with highest Cho/NAA ratio were used; control spectra were taken from voxels in NAWM (wherever possible contralateral to the tumor).
Using all voxels from NAWM a threshold for 2HG detection was calculated using two approaches : i) the 90th percentile values of 2HG/Cre and 2HG/NAA; and ii) the 90% confidence intervals (CI) for 2HG/Cr and 2HG/NAA. For comparison, a conventional threshold for 2HG detection based on CRLB% was also used – it assumed that 2HG was actually detected/detectable if relative CRLB% was less than 30%.
Statistical Analysis
2HG estimates are reported as mean and standard error of the mean (SEM). The IDH mutant (IDH1/IDH2) tumour voxels were compared to the IDH wild-type (WT) tumour voxels using a two-sample t-test with unequal variances.
RESULTS
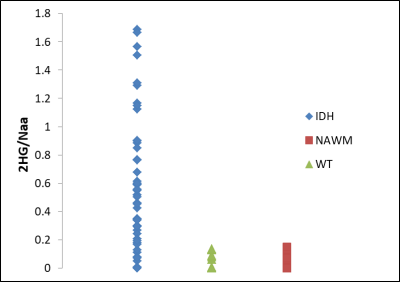
Figure 1 and Figure 2 show an edited and a non-edited spectra from an IDH-mutant patient and from a patient with IDH-WT respectively.Figure 3 presents 2HG/NAA values for lesion voxels from the IDH group and WT group, as well as NAWM voxels from all patients. Mean +/- SEM for 2HG/Cr and 2HG/NAA for the IDH-mutant, IDH-WT glioma voxels are shown in Table 1.
In our study, the derived 90th percentile thresholds were 0.115 for 2HG/NAA and 0.144 for 2HG/Cr.
The 90% CI thresholds were 0.048 for 2HG/NAA and 0.092 for 2HG/Cr.
Sensitivity and specificity derived using the aforementioned threshold criteria are given in Table 2. For comparison, sensitivity/specificity rates using the conventional 30% CRLB cutoff are also shown.
The results suggest that both 90th percentile and 90% CI thresholding methods provide similar sensitivity and specificity. Notably, improved sensitivity is achieved with our proposed method compared to the conventional approach using (30%) CRLB percentage.
DISCUSSION AND CONCLUSIONS:
Based on our overall results, 3D MEGA-LASER provides a valuable non-invasive diagnostic tool for IDH mutation status detection.In our patients, the proposed thresholding approaches for 2HG detection in the tumour voxels provided increased sensitivity and specificity as compared with the ‘classical’, rather ‘universal’ cutoff thresholds based on CRLB% relative error.
An important secondary outcome in our study was the diagnostic value of 2HG/NAA ratios from the edited spectra as a surrogate biomarker to assess the IDH mutation status. Our results indicate the need to provide individually tailored thresholds in our patient cohorts with the caveat that these might be susceptible to particular sequences, post-processing implementations and MR scanner settings.
Further studies are required to refine the calculations and confirm generalizability of our proposed thresholding concept.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 667510 and the Department of Health’s NIHR-funded Biomedical Research Centre at University College London.
This study is supported by the National Institute of Health Research Biomedical Research Council, UCL Hospitals NHS Trust.
EDV is supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z]
References
1.Bisdas S., Chadzinski GL, Braun C, et al. MR spectroscopy for in vivo assessment of the oncometabolite 2-hydroxyglutarate and its effects on cellular metabolism in human brain gliomas at 9.4T. J Magn Reson Imaging 2016 44(4): 823-33
2.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas.,J Clin Oncol. 2009;27(25):4150–4154
3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol.2016;131(6):803–820.
4. Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglu-tarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med.2012;4(116):116ra4.
5. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with glio-mas. Nat Med. 2012;18(4):624–629
6. Berrington A, Voets NL, Plaha P et al. Improved localisation for 2-hydroxyglutarate detection at 3T using long-TE semi-LASER. Tomography 2016 2(2) 94-105.
7. Kreis R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn Reson Med 2016 75(1) 15-8
8. Bogner W, Gagowski B, Hess AT, et al. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRS. Neuroimage 2014 103 290-302
Figures