0389
Simultaneous 3D proton MRF and sodium MRI1Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Sackler Institute of Graduate Biomedical Sciences, NYU Langone Health, New York, NY, United States, 3Center for Advanced Imaging Innovation and Research (CAI2R), NYU Langone Health, New York, NY, United States
Synopsis
In this work, we present a 3D sequence that can simultaneously capture quantitative 1H density, T1, T2, B1+ maps and a 23Na image of the whole head in a reasonable scan time (~10 min). The gradient momenta are strategically distributed to simultaneously acquire a full-radial trajectory for proton and a center-out radial trajectory for sodium in one single readout. A sodium SNR comparison, verification of the proton multi-parametric maps, and in-vivo results are shown.
Introduction
Sodium ions (Na+) play an important role in cell homeostasis. Consequently, metabolic information that is hard to access with proton MRI, may be observed with sodium MRI [1]. However, sodium MRI is challenged by its low intrinsic SNR and the associated obstacles, i.e. low resolution, long scan time, the necessity of accompanied proton scans for anatomical information. By acquiring the sodium image simultaneously during a proton magnetic resonance fingerprinting (MRF) scan [2], metabolic information can be collected at without lengthening the total exam time. Previously, we showed a proof-of-principle 2D implementation, which was still too slow for whole brain coverage (2min per slice, ~88min for a whole brain) [3]. In this work, we present a more advanced 3D implementation, which can simultaneously capture quantitative 1H density (PD), T1, T2, B1+ maps and a 23Na image of the whole head in a reasonable scan time (~10min).Methods
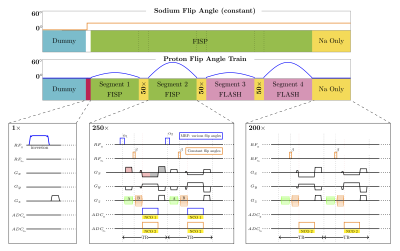
The 3D sequence is implemented using a stack-of-stars sampling scheme [4]. In each TR, a none-selective excitation (square pulse) for 1H and 23Na are played in sequence, followed by one simultaneous readout for both nuclei (Fig.1). Since proton and sodium have different gyromagnetic ratios, different partition gradient momenta are necessary to encode the same 3D slab (GNa≈ γH/γNa·GH).We strategically divided the partition gradients in two parts. Part “A”, played between 1H pulse and 23Na pulse, can only be “seen” by the proton spins and won’t affect the sodium signal. Part “B” played after 23Na pulse, effects both nuclei. Consequently, the 1H partition is defined by A+B and the 23Na partition by B.The signal from both nuclei is acquired simultaneously using the same read-out gradient. We used center-out radial trajectory for sodium acquisition to minimize the TE, but full radial trajectory for proton to reduce under-sampling artifacts in the MRF maps. Similar to our partition strategy, we inserted the 1H pre-phaser between the 1H and 23Na excitation.
To encode the T1, T2, PD and B1+ for proton MRF, in each partition (1 shot), 1000 TRs with various flip angles (FA) are played out in 4 segments (250 TRs) with a 50-TR delay in between. A 200-TR delay is added at the end for the proton magnetization to partially recover. For the sodium MRI, a constant FA (20°) was used for all TRs (including during delays for proton MRF).
All experiments were performed at 7T (MAGNETOM, Siemens, Erlangen, Germany) using a 16-channel-Tx/Rx dual-tuned coil developed in-house [5]. The received chain was modified as described in Meyerspeer et.al[36]. All images were reconstructed in MATLAB (Mathworks, Natick, MA, USA) using the Fessler NUFFT toolbox [7].
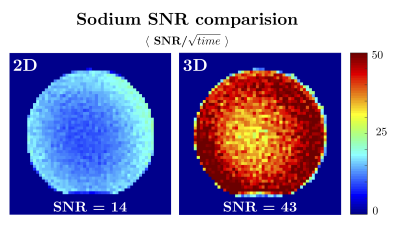
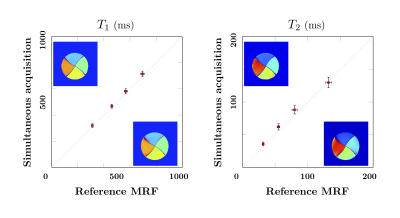
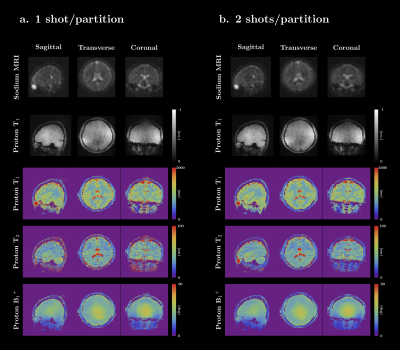
A spherical phantom (Siemens, diameter 16cm), was used to compare the scan-time normalized sodium SNR between the 2D and 3D implementations ($$$SNR/\sqrt{scan time}$$$)[8]. To capture the whole head, when the slice thickness is 3.8mm, 44 slices/partitions are needed (2D scan-time 88min, 3D scan time 10min). In addition, a four-compartment cylindrical phantom (1H T1: 400~900ms, 1H T2: 40~160ms, [Na+]: 30~150 mmol) was used to verify the accuracy of the multi-parametric 1H maps. A previously published MRF sequence was used as a reference [9]. An in-vivo brain scan was performed. The 3D sequence parameters for all phantom experiments were as follows: 1H 208×208/23Na 55×55 matrix, 1H 1×1 mm2/23Na 3.8×3.8mm2 in-plane resolution, 3.8mm slice thickness, 44 slabs, 1 shot per slab, total scan time 10min, except for the T1/T2 validation experiment where 2 shots were used (20min). The 2D experiments used the same in-plane resolution, but 5 mm slice thickness due to gradient strength limitations, and 12 shots. Total scan time for 1 slice was 2min. The in-vivo scan used the same parameters as the phantom scans, including both 1 shot and 2 shots dataset.
Results & Discussion
The 3D implementation demonstrated a 179% improvement in SNR per unit time (Fig 2), enabling coverage of the whole head in less than 10min. Excellent correlation was found between the T1 and T2 maps obtained with the simultaneous 3D and reference method (Fig 3). However, compared to our original 2D implementation, our new 3D version acquires far fewer radial samples per slice. In practice that means that, even though the 1H signal is collected using full radial readouts, our 3D MRF data has a 6× higher in plane acceleration. Consequently, residual streak-like artifacts are starting to show in the maps. It may be possible to mitigate these artifacts through optimization of the MRF flip angle train [10], a modified sampling trajectory [11], or reconstruction techniques [12].Figure 4 shows the 3D results from the in-vivo experiment. All sodium and proton data are acquired simultaneously in one scan and are perfectly co-registered. The sodium 3D data has isotropic resolution (3.8×3.8×3.8mm3) which generate nice images for all three planes. However, the proton T1 and T2 maps only has higher in-plane resolution (1×1mm2) on the sagittal slice, but a bit low resolution on other planes due to the thick slab (3.8mm). The under-sampling artifacts in the proton maps are significantly reduced when using 2 shots, underlining the need for more advanced reconstruction techniques and optimized flip-angle trains.
Conclusion
Our new 3D implementation provides an efficient solution for simultaneous volumetric 1H MRF and 23Na MRI.Acknowledgements
The research reported in this publication was supported by the NIH/NIBIB grant R01 EB026456, and performed under the rubric of the Center for Advanced Imaging Innovation and Research, a NIBIB Biomedical Technology Resource Center (P41 EB017183).References
[1] Madelin G, & Regatte R R. Biomedical applications of sodium MRI in vivo. Journal of Magnetic Resonance Imaging, 2013;38:511-529.
[2] Ma D, Gulani V, Seiberlich N, Liu K, Sunshine J L, Duerk J L, & Griswold M A. Magnetic resonance fingerprinting. Nature, 2013;495:187–192.
[3] Yu, Z., Madelin,G.1,2, Sodickson, D. K., & Cloos, M. A. (2019). Simultaneous proton MR fingerprinting and sodium imaging. Proc. Intl. Soc. Mag. Reson. Med. 27 (2019)
[4] Block KT, Chandarana H, Milla S, et al. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J Korean Soc Magn Reson Med. 2014;18(2):87-106. doi:10.13104/jksmrm.2014.18.2.
[5] Bili Wang, Martijn Cloos, Ryan Brown, Guillaume Madelin, Daniel K. Sodickson, Bei Zhang. Dual-Tuned 16-Channel TxRx Array for Sodium & Proton Brain Imaging at 7T. 2019.
[6] Meyerspeer M, Magill A W, Kuehne A, Gruetter R, Moser E, & Schmid A I. Simultaneous and interleaved acquisition of NMR signals from different nuclei with a clinical MRI scanner. Magnetic resonance in medicine, 2016;76:1636-1641.
[7] Fessler J A, & Sutton B P. Nonuniform fast Fourier transforms using min-max interpolation, IEEE Transactions on Signal Processing, 2003;51:560-574.
[8] Kellman P, & McVeigh E R. Image reconstruction in SNR units: a general method for SNR measurement. Magnetic resonance in medicine, 2005;54:1439-1447.
[9] Cloos M A, Knoll F, Zhao T, Block K T, Bruno M, Wiggins G C, & Sodickson D K. Multiparametric imaging with heterogeneous radiofrequency fields. Nature communications 2016;7:12445.
[10] Assländer J, Novikov D S, Lattanzi R, et al. Hybrid-state free precession in nuclear magnetic resonance[J]. Communications Physics, 2019, 2(1): 73.
[11] Körzdörfer G, Pfeuffer J, Kluge T, et al. Effect of spiral undersampling patterns on FISP MRF parameter maps[J]. Magnetic resonance imaging, 2019.
[12] Assländer J, Cloos M A, Knoll F, et al. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting[J]. Magnetic resonance in medicine, 2018, 79(1): 83-96.
Figures