0380
Documentation of Anti-glutamatergic Effect of N-Acetylcysteine Treatment with 1H MRS Monitoring of Cortical Glutathione and Glutamate In Vivo1Radiology, Weill Cornell Medicine, New York, NY, United States, 2Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Neurology, Weill Cornell Medicine, New York, NY, United States
Synopsis
N-acetylcysteine (NAC), a glutathione (GSH) synthesis precursor, is thought to have anti-glutamatergic properties for which direct in vivo evidence is lacking. In this study, the postulated anti-glutamatergic properties of NAC were investigated by using 1H MRS to monitor changes in brain levels of both GSH and glutamate (Glu) in response to 4 weeks of NAC supplementation in patients with chronic fatigue syndrome (CFS) and healthy volunteers (HV). Following NAC treatment, GSH levels increased significantly in CFS and numerically in HV, while Glu decreased significantly in both groups compared to baseline – a finding that supports NAC as an anti-glutamatergic agent.
INTRODUCTION
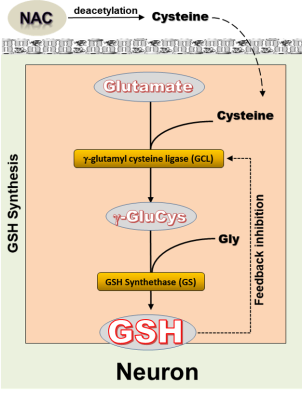
Deficits of brain glutathione (GSH), the primary antioxidant in living tissue are postulated to be implicated in various neurological and neuropsychiatric disorders. However, due to the poor blood-brain barrier permeability of GSH, direct supplementation is ineffective in elevating its brain levels. Therefore, there has been great interest in investigating N-acetylcysteine (NAC), as a prodrug that can be deacetylated to supply cysteine (Cys), the rate-limiting substrate in GSH synthesis (Figure 1), which can cross the BBB. Once inside the cell, Cys combines with glutamate (Glu) to initiate GSH synthesis in a reaction that is catalyzed by γ-glutamyl cysteine ligase (GCL), the rate-limiting enzyme (Figure 1). Likely due to the GCL-catalyzed reaction of NAC-supplied Cys with Glu to spur GSH synthesis, NAC is believed to have anti-glutamatergic properties. However, direct in vivo evidence of NAC as an anti-glutamatergic agent is currently lacking. In this study, we used 1H MRS to monitor changes in brain levels of both GSH and Glu in response to 4 weeks of daily supplementation with NAC in patients with chronic fatigue syndrome (CFS) and matched healthy volunteer (HV) subjects. Having previously shown [1] that 4 weeks of NAC supplementation significantly increased brain levels of GSH in CFS, while eliciting only a numerically non-significant increase in mean GSH in HV, we postulated that the effect of NAC on Glu levels would be opposite to that observed for GSH, i.e., a decrease in CFS patients and, possibly, also in HV.METHODS
Subjects: Participants recruited for this study consisted of 12 medication-free patients with CFS, diagnosed according to the CDC criteria [2], and 12 HV subjects, who served as the normal comparison group.NAC Supplementation: To investigate the effects of dietary NAC supplementation on cortical GSH and Glu levels, each subject underwent a 1H MRS scan at baseline. Then a 4-week supplement of 900mg NAC tablets was provided, to be taken 2 per day for a daily NAC dose of 1800mg. Finally, each subject was brought back after 4 weeks for the post-NAC 1H MRS scans to determine the effect of the treatment on cortical GSH and Glu levels.
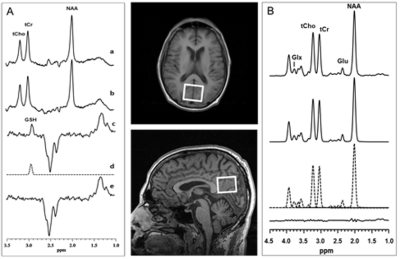
Brain 1H MRS: In vivo cortical GSH spectra were recorded in 15 min on a 3.0 T GE MR system from a 3.0x3.0x2.0-cm3 occipital cortex voxel (Figure 2) using the standard J-editing technique (Figure 2A), with TE/TR 68/1500ms and 290 interleaved excitations (580 total) as recently described [3]. Levels of Glu, uncontaminated with glutamine, were obtained from the same voxel in 6 min using the CT-PRESS sequence [4] (Figure 2B), with TE/TR 139ms/1500ms, and 129 chemical shift encoding steps in increments of 0.8ms in t1 dimension. Peak areas for both GSH and Glu were derived by frequency-domain fitting of the recorded spectra.The resulting peak areas were then expressed as ratios relative to the area of the unsuppressed tissue water (W) signal in the same voxel [3].
RESULTS
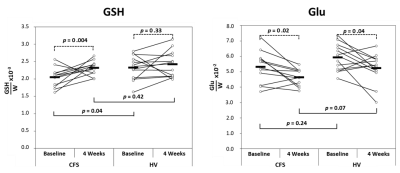
GSH Levels (Figure 3): As previously reported [1], we found that within the CFS group, the effect of 4 weeks of NAC supplementation was to increase cortical GSH levels significantly compared to baseline (p=0.004), while within the HV group mean GSH levels increased numerically without reaching statistical significance relative to baseline (p=0.33). Between the CFS and HV groups, there was a significant GSH deficit at baseline in CFS (p=0.04) relative to HV, which vanished following 4 weeks of NAC (p=0.42), indicating normalization of GSH levels in CFS with NAC.Glu Levels (Figure 3): Within both the CFS and HV groups, the effect of 4 weeks of NAC supplementation was to decrease cortical Glu significantly compared to baseline (p=0.02 and p=0.04, respectively). Between the CFS and HV groups, mean Glu levels did not differ at baseline (p=0.24). However, following 4 weeks of NAC, there was a trend-level lower Glu in CFS than in HV (p=0.07), indicating a larger Glu decrease in CFS than in HV after NAC.
DISCUSSION AND CONCLUSION
This study has documented, for the first time, that in situ GSH synthesis following NAC supplementation is accompanied by a significant decrease of brain Glu, supporting the postulated anti-glutamatergic property of NAC. The decrease in total Glu levels at 4 weeks suggests that there might be a net increase in the consumption of intracellular Glu reserves in the GCL-catalyzed reaction that combines NAC-supplied Cys with Glu as the first and rate-limiting step in GSH synthesis (Figure 1). Interestingly, there appears to be a degree of consistency between the changes in GSH and Glu in the CFS and HV groups: 4 weeks of NAC numerically increased GSH in the HV group without reaching statistical significance, in apparent consistency with a correspondingly smaller decrease in Glu at 4 weeks. By contrast, the larger and statistically significant increase in GSH after 4 weeks of NAC appeared to elicit a correspondingly larger Glu decrease in the CFS group. In summary, the results of this study support the role of NAC as an anti-glutamatergic agent, which seems to modulate Glu levels by increasing the availability of intracellular Cys that then combines with Glu to initiate GSH synthesis, leading to a lowering of total Glu levels as measured with MRS.Acknowledgements
Support for this study was provided by NIH/NINR 5R21NR01365001.References
[1] Weiduschat N, et al. Proc Intl Soc Magn Reson Med 2017; 25: Abstract #4553.
[2] Fukuda K, et al. Ann Intern Med 1994; 121: 953-959.
[3] Shungu DC, et al. NMR Biomed 2012; 25:1073-87
[4] Mayer D and Spielman DM. Magn Reson Med 54, 439-442.
Figures