0373
Adiabatic multiband inversion for simultaneous acquisition of 1H MR spectra from two voxels in-vivo at very short echo times1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 2Center for Stroke Research Berlin, Charité-Universitätsmedizin, Berlin, Germany
Synopsis
In this work, a novel 1H MR spectroscopy sequence is proposed that provides the advantages of single voxel spectroscopy, such as high spectral bandwidth, a narrower point spread function, shorter measurement time and larger signal-to-noise-ratio, as compared to spectroscopic imaging while exciting more than one voxel. A multi-band adiabatic RF pulse was implemented into a SPECIAL sequence to simultaneously acquire the signal of two disjunct voxels at short echo times. The overlapping signal was decomposed using the SENSE algorithm. The new sequence was validated using a two-compartment phantom and its feasibility for in-vivo application is demonstrated at 7 T.
Introduction
While single voxel spectroscopy (SVS) provides an excellent metabolite signal-to-noise-ratio (SNR) and a narrow point spread function1 at short measurement times, this technique allows measuring signal from only a single, small region2.Furthermore, several metabolites in the brain including glutamate and glutathione, have short T2 times3 and, therefore, 1H magnetic resonance spectroscopy (MRS) acquisition techniques with short echo times (TE) are required to reliably determine their concentrations. Short TEs, that become even more important at ultra-high fields (UHF) due to further decreased T2 values, can be achieved with the spin-echo full-intensity acquired localization (SPECIAL)4,5 sequence.
In this work, we extend the limited spatial coverage of SPECIAL and present '2SPECIAL', a novel, SPECIAL-based simultaneous two voxel acquisition technique. The method was investigated and validated in a two-compartment phantom and its feasibility for in-vivo measurements is demonstrated.
Methods
All measurements were performed on a 7T Scanner (Magnetom 7T, Siemens, Erlangen, Germany) using a 1Tx/32Rx head coil (NOVA Medical, Wilmington, USA), unless specified differently.Multiband Hyperbolic Secant Pulse
Two frequency shifted hyperbolic secant (HS) adiabatic pulses6 were superimposed7 to generate a multi-band (MB) HS pulse. To ensure the pulse is executed correctly by the scanner, the MB-HS pulse was measured with a pickup coil and an RSPduo receiver (SDRPlay, Hampshire, UK) and compared to the theoretical magnitude of the SB and MB pulse. Furthermore, Bloch simulations of the theoretical SB and MB pulse were performed.
2SPECIAL sequence
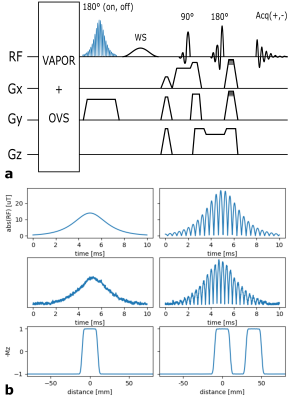
The adiabatic MB pulse was then inserted into the SPECIAL sequence to invert two slices simultaneously (Fig.1a). Subsequent excitation and refocusing in orthogonal directions in combination with the add/subtract scheme of SPECIAL enables the measurement of spectral signal from two spatially distinct voxels. Like with conventional SPECIAL, outer volume saturation (OVS) and water suppression (VAPOR) were applied before the adiabatic inversion.
SENSE decomposition
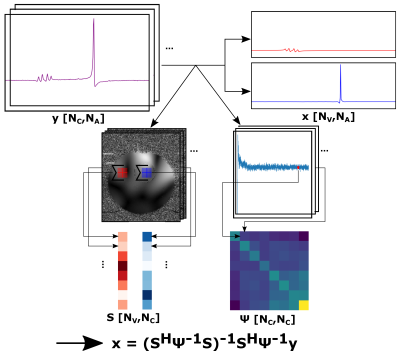
The SENSitivity Encoding (SENSE) formalism8,9 was applied in Python10 (Version 3.6) to decompose the joint signal into the two different voxels (Fig.2). The noise covariance per channel combination was calculated by taking one fixed point in the noise of the spectral measurement for each coil and average. Coil sensitivities were derived from a fast gradient echo sequence.
Phantom measurements
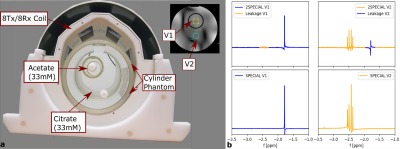
MB acquisition and reconstruction were validated using an in-house-built 8Tx/8Rx head coil with fixed Tx phases and a cylindrical two-compartment phantom (Fig.3a). The inner cylinder was filled with 33mM acetate, the surrounding compartment with 33mM citrate. To validate the SENSE decomposition, initial MPRAGE11 images were acquired to obtain the coil profiles and to position the voxel. First, the spectra of two spatially separated voxels, one in each compartment, were acquired using SPECIAL (NA=32,TE/TR=9/6500ms,VOI=(20mm)³,data points=2048,acquisition bandwidth=4000Hz). 2nd order B0 shimming12 and power adjustment were conducted for each voxel, individually. Then, the same two voxels were acquired by 2SPECIAL (same parameters, voxel distance=40mm) using a trade-off for power adjustments and B0 shim between voxels. The signal was SENSE decomposed and signal leakage between voxels was quantified by integration of the respective leak signal, normalized to the same-metabolite signal in the origin voxel (Fig.3b).
In-vivo scan
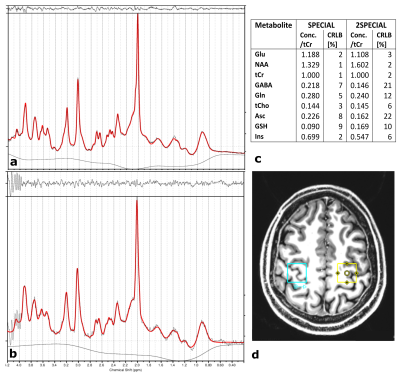
In-vivo measurements, approved by the local ethics board, were performed in one healthy volunteer. An MP2RAGE13 image was acquired for determination of coil sensitivity profiles and positioning of the voxels in the left and right motor cortex14(Fig.4). A SPECIAL (NA=64,TE/TR=9/6500ms,VOI=(20mm)³,data points=2048,acquisition bandwidth=4000Hz) measurement for each individual voxel, and a 2SPECIAL (same settings,voxel distance=60mm) acquisition were performed. The resulting signals were zero-filled, apodised and zero order phase corrected. LCModel15 was used to estimate the concentration of the metabolites.
Results and Discussion
As shown in Fig.1b, the measured RF signal shapes agree with the theoretical ones. Furthermore, Bloch simulations of the resulting pulse profile of the theoretical HS pulse reveal correct slice thickness and slice locations. It is therefore expected that the actual slice thickness and slice location match the simulated ones.Fig.3b demonstrates the feasibility to separate the spectra from the two different phantom voxels. However, a small signal leakage of 4.1% in the first voxel (V1) and 3.2% in the second voxel (V2) is visible. This indicates imperfections of the RF pulses that are not considered in the decomposition yet.
Qualitatively, the fitted signal obtained by the SPECIAL and 2SPECIAL in-vivo measurements reveal similar line shapes, however a slightly increased noise level can be observed in the 2SPECIAL measurement. The culprit for this, as well as the spurious signal around 4ppm, is likely the trade-off in power adjustments and B0 shim between the voxels. Quantitatively, the determined concentrations (see Fig.4c) are similar for tCr, tCho, Glu, GSH, Ins and NAA with maximum increase in the Cramér-Rao-Lower-Bounds (CRLBs) of 4% but show difference in the CRLBs of more than 7% for metabolites with low signal intensities, such as GABA, Gln, and Asc (Fig.4a/b), which is likely caused by leaked signal with potential slight frequency shifts.
Conclusion
We have successfully demonstrated that 2SPECIAL, a SPECIAL sequence with a multi-band HS inversion pulse, can be effectively used to acquire high quality MR spectra simultaneously from two voxels in-vivo and reliably quantify metabolite content, while cutting the effective measurement time in half. Such a technique would greatly facilitate spectroscopic measurements in a lesion and its contra-lateral control region.Acknowledgements
No acknowledgement found.References
[1] Skotch, A., Jiru, F., Bunke, J. (2008), Spectroscopic imaging: basic principles. Eur J Radiol., 67: 230-239.
[2] Oz, G., Alger, J. R., Barker, P. B., Bartha, R., Bizzi, A., Boesch, C. et al. MRS Consensus Group (2014), Clinical proton MR spectroscopy in central nervous system disorders. Radiology, 270(3): 658–679.
[3] Marjańska, M., Auerbach, E. J., Valabrègue, R., Van de Moortele, P. F., Adriany, G., Garwood, M. (2012), Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed., 25(2): 332-339.
[4] Mlynárik, V., Gambarota, G., Frenkel, H. and Gruetter, R. (2006), Localized short‐echo‐time proton MR spectroscopy with full signal‐intensity acquisition. Magn. Reson. Med., 56: 965-970.
[5] Mekle, R., Mlynárik, V., Gambarota, G., Hergt, M., Krüger, G., Gruetter, R. (2009), MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn. Reson. Med., 61: 1279-85.
[6] Tannús, A. and Garwood, M. (1997), Adiabatic pulses. NMR Biomed., 10: 423-434.
[7] Barth, M., Breuer, F., Koopmans, P. J., Norris, D. G. and Poser, B. A. (2016), Simultaneous multislice (SMS) imaging techniques. Magn. Reson. Med., 75: 63-81.
[8] Landheer, K., Sahgal, A., Das, S., Graham, S. (2015), Constrained Source Space MR Spectroscopy: Multiple Voxels, No Gradient Readout. Am J Neuroradiol., 36(8): 1436-43.
[9] Boer, V. O., Klomp, D. W., Laterra, J., Barker, P. B. (2015), Parallel reconstruction in accelerated multivoxel MR spectroscopy. Magn. Reson. Med., 74: 599-606.
[10] van Rossum, G. (1995), Python tutorial, Technical Report CS-R9526, Centrum voor Wiskunde en Informatica (CWI).
[11] Mugler, J.P., Brookeman, J.R. (1991), Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging, 1: 561-570.
[12] Nassirpour, S., Chang, P., Fillmer, A., Henning, A. (2018), A comparison of optimization algorithms for localized in vivo B0 shimming. Magn. Reson. Med., 79(2): 1145-56.
[13] Marques, J.P., Kober, T., Krueger, G., van der Zwaag, W., van de Moortele, P.F., Gruetter, R. (2010), MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage., 49(2): 1271-78.
[14] Yousry, T. A., Schmid, U. D., Alkadhi, H., Schmidt, D., Peraud, A., Buettner, A., Winkler, P. (1997), Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain., 120(1): 141-157.
[15] Provencher, S. W. (1993), Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med., 30: 672-679.
Figures