0372
Towards sub-microlitre MRS in the mouse brain in vivo at ultra-high field1Core Facility Small Animal Imaging, Ulm University, Medical Center, ulm, Germany, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3German Center for Neurodegenerative Diseases (DZNE), Ulm, Germany
Synopsis
Several pathological conditions affect only a small volume of the cortex (such as the motor cortex in amyotrophic lateral sclerosis) and its characterization in mouse models is made impossible by the interference of normal, nearby cortical tissue. A sub-microlitre preclinical MRS technique was successfully implemented to detect subtle changes of the neurometabolite concentrations in three cortical areas. Employing LASER together with using cryogenically cooled RF coils significantly reduces the acquisition time to enable sub-microlitre MRS acquisition. Our findings demonstrate that neurochemical profiles of individual cortical brain regions can be reliably collected in pre-clinically feasible scan times.
Purpose
Proton magnetic resonance spectroscopy is a powerful non-invasive method to measure neurochemicals in vivo. However, due to the low intensities of metabolite signals, relatively large volume-of-interest (VOI) is often used together with a large number of averages to increase the signal-to-noise ratio (SNR). This poses a challenge for investigating specific region since large VOI will usually contain contributions from several regions. To provide in vivo metabolomic profiles from specific substructure in cortical areas, it is therefore important to reduce partial volume effect. This can only be achieved by using sub-microlitre VOI size. However, small VOI suffers from low SNR at the expense of longer acquisition time. In the mouse brain, VOI sizes generally used are between 5 to 10 µL (1). To our knowledge, the smallest VOI reported to date was 1.8 µL in adult mice at 11.7T (2). The goal of the current study is to show the feasibility of acquiring sub-microlitre single-voxel 1H-MRS data in the mouse brain in a reasonable time by taking advantage of several advances in the MRS field. We use the LASER sequence (3) with a cryoprobe at an ultra-high field of 11.7T. LASER is advantageous as the sequence retains full signal echo with increased T2 relaxation times and reduced J-modulation at relatively short echo-time (TE) (4). On the other hand, the SNR increases by a factor of 2-3 with cryoprobe compared to room-temperature coil (5).Methods
Experiments were performed at a dedicated ultrahigh field 11.7T small animal system (117/16 USR BioSpec, AVANCE III, ParaVision 6.01, Bruker BioSpin, Ettlingen, Germany) equipped with a 9 cm inner diameter self-shielded gradient coil insert providing 750 mT/m maximal strength in 80 μs rise time. Cryogenically cooled 2-element phased-array transmit/receive coil was employed for excitation and signal reception. A home-built head restrainer was used to properly immobilize the animal's head during measurements, ensuring stability and reproducibility of the experimental setup. Volume-of-interests (VOI) were planned based on T1-weighted multi-slice FLASH (TR/TE = 193/5ms, flip angle 17.5°) images. Field homogeneity was adjusted for each investigated region using a field-map based approach (MAPSHIM). A short echo-time LASER sequence (3) (TR/TE: 5000/16.75ms, 10 kHz spectral width, 4096 data points and 386 averages) combined with VAPOR water suppression was used (6). In vivo 1H MR spectra were acquired from 0.729mm3 (0.9x0.9x0.9 mm3) volume located in the primary motor cortex, primary somatosensory cortex, and olfactory bulb of three adult female C57BL/6. Single-shot data were frequency and phase corrected prior to summation (7). Unsuppressed water signal was used as an internal reference as well as for eddy current, zero-order and first-order phase correction. Absolute metabolite concentrations were derived with LCModel (spectrum fitted from 0.5–4.2 ppm) (8). Metabolite concentrations with a Cramér–Rao lower bound (CRLB) ≤50% in at least half of the spectra in each brain region were used for statistical analysis. The sum of metabolites was reported (e.g. tCr, tCho) when a high correlation existed between two metabolites (r<-0.5). Non-parametric Kruskal-Wallis test was used to assess the mean metabolites concentration in the three regions, Dunn’s multiple comparison test was used for multiple p value correction.Results and Discussion
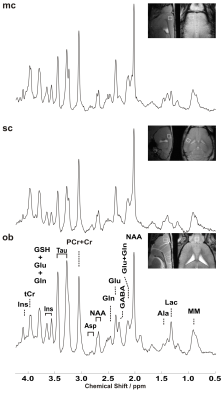
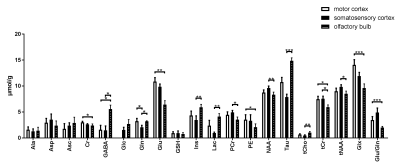
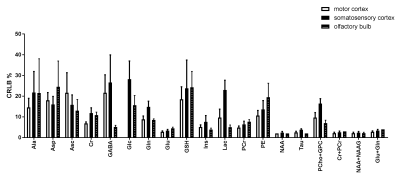
By employing a 1.3ms (13857Hz bandwidth) AFP pulses (HS1-R20) a minimum echo time of 16.75 ms was realized in LASER. In addition, the chemical shift displacement error (CSDE) was minimized (3.6%/ppm) as a result of using high bandwidth AFP pulses together with strong gradient. This consequently translates into 0.1 mm translation in X-, Y- and Z-dimensions for the 3 ppm range. Representative water-suppressed in vivo proton MR summed spectra of the investigated mouse cortical regions clearly show the well-resolved resonances of numerous cerebral metabolites signals (Fig. 1), obtained with sufficiently consistent spectral quality. The average full width at half-maximum found by LCModel was 0.02±0.00 ppm (10.0±0.0 Hz) in the motor cortex, 0.02±0.01 ppm (10.0±5.0 Hz) in the somatosensory cortex, and 0.02±0.00 ppm (10.0±0.0 Hz) in the olfactory bulb. Corresponding SNRs were 15.7±1.25, 12.8±1.60 and 12.5±0.76, respectively. The high spectral quality achieved over the entire chemical shift range (0.5–4.2 ppm) ensured reliable and reproducible quantification of each of the brain metabolites. A detailed comparison of cortical areas neurochemical profiles of C57BL/6 mice is shown in Fig. 2. The average Cramér–Rao lower bound (CRLB) corresponding to the fitted metabolites are shown in Fig. 3. Average CRLB for Glx, tNAA, tCr and NAA was ≤ 3% and for many of the other major metabolites was ≤ 10% in all regions, proving the reliability of the quantification of the brain metabolites. Despite the fact that scyllo-inositol and N-acetylaspartylglutamate (NAAG) signals were incorporated into the basis set of LCModel as a model component, corresponding quantification was not reliably possible.Conclusion
To our knowledge, this is the first successful implementation of a sub-microlitre preclinical MRS in vivo. LASER spectra were reproducible and in good agreement with neurochemical profiles reported in previous studies (1). Employing LASER together using cryogenically cooled RF coils significantly reduces the acquisition time to enable sub-microlitre MRS acquisition. Our findings demonstrate that neurochemical profiles of individual cortical brain regions using sub-microlitre VOI size can be reliably collected in pre-clinically feasible scan times.Acknowledgements
NIH grants: P41 EB027061, P30 NS076408References
1. Tkác I, Henry PG, Andersen P, Keene CD, Low WC, Gruetter R. Highly resolved in vivo 1H NMR spectroscopy of the mouse brain at 9.4 T. Magn Reson Med. 2004 Sep;52(3):478-84.
2. Santin MD1, Valabrègue R, Rivals I, Pénager R, Paquin R, Dauphinot L, Albac C, Delatour B, Potier MC. In vivo 1H MRS study in microlitre voxels in the hippocampus of a mouse model of Down syndrome at 11.7 T. NMR Biomed. 2014 Oct;27(10):1143-50.
3. Garwood, M. & DelaBarre, L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 153, 155-177, (2001).
4. Deelchand DK, Henry PG, Marjańska M. Effect of carr‐purcell refocusing pulse trains on transverse relaxation times of metabolites in rat brain at 9.4 Tesla. Magn Reson Med. 2015 Jan;73(1):13-20
5. Baltes, C., Radzwill, N., Bosshard, S., Marek, D. & Rudin, M. Micro MRI of the mouse brain using a novel 400 MHz cryogenic quadrature RF probe. NMR in biomedicine 22, 834-842, (2009).
6. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med (1999) 41:649.
7. http://www.cmrr.umn.edu/downloads/mrspa
8. Provencher, Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed (2001) 14:260.
Figures