0361
Towards kinematic knee imaging with a liquid metal array1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland
Synopsis
Kinematic MR studies provide functional insights that corresponding static methods may not be able to provide. However, MR signal reception from body parts with large flexion ranges, such as the knee, can be challenging. Wearable RF coils that adapt well to a specific anatomy would offer good sensitivity and patient comfort at the same time. In the present work, we explore the practical utility of a wearable liquid metal coil. For this purpose a MR compatible knee bending setup is used. Static and kinematic imaging of a volunteer’s knee confirm sensitivity and coverage over the whole range of flexion.
Introduction
Kinematic MR studies can offer functional insights that static methods may not be able to provide1-6. However, MR signal reception from flexing body parts, such as the knee, can be challenging. Use can be made of commonly available coils, such as a clinical scanner’s body coil, surface array coils or individual surface coils. However, while allowing flexion of the joint, such setups yield suboptimal sensitivity, hamper reproducibility, and often require impractically long setup times for potential clinical application. The use of current commercial knee arrays is prevented by the impossibility of flexion of the joint.Kinematic knee studies demand a coil setup that fits well to the individual knee, optimizes sensitivity and patient comfort, and allows flexion of the joint at the same time. Several concepts for stretchable coils have been proposed in the literature7–11. One recent approach forms stretchable conductors by containing liquid metal in elastic tubes and has been demonstrated in a multiple-channel knee setup12.
In the present work, we advance this concept towards an integrated, wearable detector garment and explore its utility for kinematic imaging. For mechanical stability, reproducibility and smooth kinematic imaging we use a dynamic, MR-compatible knee support. Sensitivity and coverage of the wearable array are explored at different flexion angles and in kinematic knee imaging. To relate these result to clinical practice, an SNR comparison with a current commercial knee array is reported.
Methods
Wearable Coil ArrayEutectic Gallium Indium is liquid at room temperature. By containing it in silicone tubes (Fig. 1), coils with high electrical performance offering high stretchability are formed12. Stretchable coil elements are combined into a wearable 4-channel knee array which is created by sewing together two layers of highly elastic athletic pants. The sewing pattern generates textile casings that ensure stretchability of the entire array and approximate geometric decoupling for proper coil performance.
Knee Bending Setup
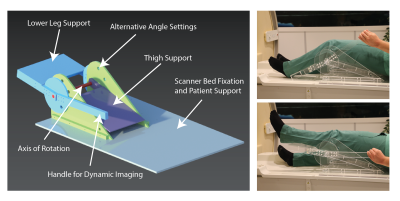
Using computer-aided design software (NX, Siemens, Germany) a knee bending setup (Fig. 2) was designed for application in a Philips 3T Ingenia system. When the volunteer is lying in feet first supine position the leg is placed inside the setup with the knee joint above the axis of rotation. The setup allows for several discrete angles of flexion of the knee and provides a handle for stepless kinematic imaging. The setup consists of MR compatible material only.
Imaging
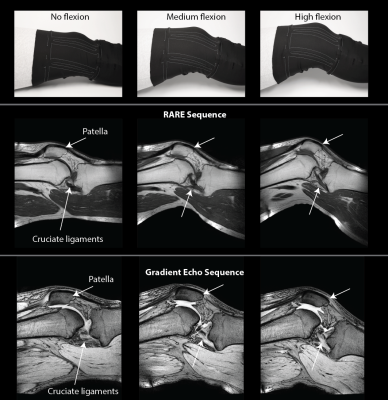
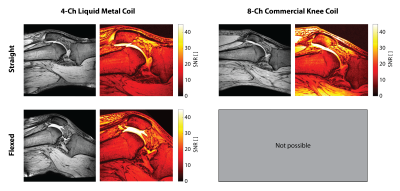
All images were acquired on a Philips 3T Ingenia system. In-vivo images of a volunteer’s knee were acquired at 3 different flexion angles (Fig. 3) using a RARE sequence (TR 523 ms, TE 9 ms, 0.6x0.69x3 mm3, 17 slices, scan duration 3:30 min) and a gradient echo sequence (TR 500 ms, TE 5.8 ms, FA 30°, 0.7x0.7x3 mm3, 24 slices, scan duration 4:28 min). SNR maps derived from the gradient echo images were calculated13 for comparison to a commercial 8-channel dedicated knee coil (Fig. 4). Kinematic imaging (Fig. 5) was performed using a SPGR sequence (TR 9.9 ms, TE 5.9 ms, FA 15°, 1x1x5 mm3, 1 slice, 30 dynamics, scan duration 1:22 min).
Results
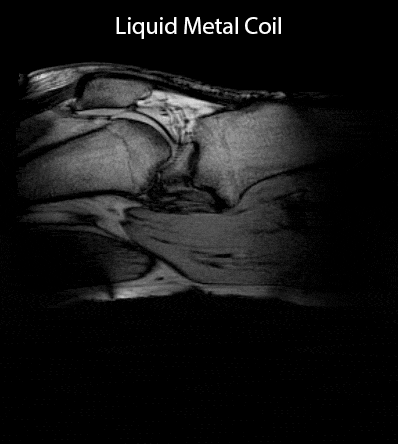
Static in-vivo images acquired with RARE and gradient echo sequences (Fig. 3) confirm sensitivity and coverage at a straight configuration of the knee as well as when the knee is flexed at different flexion angles. Rearrangement of the patella and the cruciate ligaments upon flexion of the knee are readily visible. Comparison of the 4-channel wearable liquid metal coil to an 8-channel commercial dedicated knee coil (Fig. 4) demonstrates comparable sensitivity and coverage in magnitude images. SNR comparison demonstrates comparable SNR in the center region of the knee joint, particularly the cruciate ligaments. The commercial knee coil achieves higher SNR in the surface mainly in the skin and muscles due to a higher number of coil elements. The SNR comparison demonstrates the well maintained SNR for the wearable liquid metal coil when the knee is flexed.The commercial coil mechanically prohibits flexion of the knee joint. Kinematic imaging (Fig. 5) shows good sensitivity and coverage over the whole range of flexion of the volunteer’s knee.Discussion
The results of this study demonstrate that imaging of joints in flexion and kinematic imaging is feasible with a wearable 4-channel liquid metal array. The coil provides good sensitivity and coverage for all tested flexion angles and kinematic imaging. Its SNR performance in the central region of the knee joint is comparable to a commercial dedicated knee coil and is maintained under flexion. Besides good imaging performance the wearable liquid metal coil is expected to offer greater patient comfort in kinematic imaging and shorter setup times as the coil can be put on by the patient him- or herself just like a piece of clothing.Acknowledgements
References
1. C. J. Burke et al., “Clinical Utility of Continuous Radial Magnetic Resonance Imaging Acquisition at 3 T in Real-time Patellofemoral Kinematic Assessment: A Feasibility Study,” Arthrosc. - J. Arthrosc. Relat. Surg., vol. 34, no. 3, pp. 726–733, 2018.
2. V. Mazzoli et al., “Accelerated 4D self-gated MRI of tibiofemoral kinematics,” NMR Biomed., vol. 30, no. 11, pp. 1–11, 2017.
3. A. G. D’Entremont, J. A. Nordmeyer-Massner, C. Bos, D. R. Wilson, and K. P. Pruessmann, “Do dynamic-based MR knee kinematics methods produce the same results as static methods?,” Magn. Reson. Med., vol. 69, no. 6, pp. 1634–1644, 2013.
4. F. Jia, H. Yuan, D. Zhou, J. Zhang, X. Wang, and J. Fang, “Knee MRI under varying flexion angles utilizing a flexible flat cable antenna,” NMR Biomed., vol. 28, no. 4, pp. 460–467, 2015.
5. J. A. Nordmeyer-Massner et al., “MR imaging of healthy knees in varying degrees of flexion using a stretchable coil array provides comparable image quality compared to a standard knee coil array,” Eur. J. Radiol., vol. 85, no. 3, pp. 518–523, 2016.
6. B. Zhang, D. K. Sodickson, and M. A. Cloos, “A high-impedance detector-array glove for magnetic resonance imaging of the hand,” Nat. Biomed. Eng., vol. 2, no. August, pp. 1–8, 2018.
7. J. A. Nordmeyer-Massner, N. De Zanche, and K. P. Pruessmann, “Stretchable coil arrays: Application to knee imaging under varying flexion angles,” Magn. Reson. Med., vol. 67, no. 3, pp. 872–879, 2012.
8. B. Gruber and S. Zink, “Anatomically adaptive coils for MR Imaging – A 6-channel Demonstrator Array study at 1 . 5 Tesla,” in Proc. Joint Annual Meeting ISMRM-ESMRMB, Paris, France, 2018, pp. 2–5.
9. J. Vincent and J. Rispoli, “Stretchable and Flexible Conductive-Thread Based Radiofrequency Coils for Magnetic Resonance Imaging,” Proc. Intl. Soc. Mag. Reson. Med. 27, p. 1490, 2019.
10. B. Kahraman Agir, B. Bayrambas, K. Yegin, and E. Ozturk Isik, “Wearable and stretchable surface breast coil,” Proc. Intl. Soc. Mag. Reson. Med. 27, pp. 2–4, 2019.
11. M. Varga et al., “Adsorbed Eutectic GaIn Structures on a Neoprene Foam for Stretchable MRI Coils,” Adv. Mater., vol. 29, no. 44, pp. 1–7, 2017.
12. A. Port et al., “Liquid metal in stretchable tubes: A wearable 4-channel knee array,” Proc. Intl. Soc. Mag. Reson. Med. 27, p. 1114, 2019.
13. J. A. Nordmeyer-Massner, N. De Zanche, and K. P. Pruessmann, “Mechanically adjustable coil array for wrist MRI,” Magn. Reson. Med., vol. 61, no. 2, pp. 429–438, 2009.
Figures