0349
DTI of articular cartilage as a biomarker for OA diagnosis, staging and progression in a population with early stages of the disease1Radiology, NYU Langone Health Hospital, New York, NY, United States, 2NYU Langone Health Hospital, New York, NY, United States
Synopsis
Early detection of knee osteoarthritis can be achieved by identifying early compositional changes of degenerative articular cartilage. The purpose of this case-control longitudinal study is to validate DTI as a biomarker for OA diagnosis, staging and progression in early stages of the disease. 60 patients with incipient OA (KL1) underwent 3 visits (baseline, 1.5 year and 3 years follow up). Clinical evaluation, Xray and MRI was performed. Positive correlation was demonstrated with morphological changes (KL and WORMS score). In addition, DTI showed changes in the follow up at 1.5 years that were not apparent in clinical MRI.
Introduction
There is a need for clinical biomarkers for OA diagnosis, staging and disease progression.1 Such biomarkers can impact patient management and lead to more effective clinical trials to evaluate novel therapies. Several MRI biomarkers for articular cartilage that have shown potential as subrogate measures of cartilage composition.2 However, we are still lacking a biomarker truly specific for collagen.2 DTI was introduced as a biomarker specific for proteoglycan content and collagen structure and has demonstrated to be a promise for OA diagnose OA.3-6 The goal of this study is to validate DTI of articular cartilage at 3T as a biomarker for OA diagnosis, staging and progression in a population with early stages of the disease and high likelihood of short-term progression.Methods
Study designWe identified a patient population using data form the Osteoarthritis Initiative (OAI). Knees with incipient OA (Kellgren-Lawrence [KL] score 1) form patients with established tibiofemoral OA (KL≥2) in the other knee have a 23% progression rate in KL score within 3-years. Thus, we recruited n=60 patients (male/female=26/34, age=63 y, BMI=X kg/cm2) with unilateral OA (symptomatic and KL≥2 and incipient OA in the other knee. 13 patients volunteered for a MRI of the contralateral knee (n=11 KL2, n=2 KL 3). Age-matched controls (n=12) were included.
MRI and Imaging processing
The study included 3 visits. At baseline examination, participants underwent clinical evaluation and Xray for diagnose. We perform MRI at baseline (n=60) and 1.5 years (n=42 completed so far). MRI protocol included clinical knee protocol, DTI measured with a RAISED sequence (TE/TR=35/1500 ms, 105 spokes/image, 6 directions, b-values=0,300 s/mm2) and T2 with a multi-echo spin -echo sequence (TE/TR=10.5/3000 ms, echo-train length 12, matrix=256×208, iPat=2). Diffusion images were reconstructed using a non-linear motion correction, algorithm and diffusion parameter maps mean diffusivity (MD) and fractional anisotropy (FA) were calculated. MRI were segmented in b0 image and overlaid to T2 measurements. Segmentation was divided into regions: femoral trochlea, lateral femoral condyle, medial femoral condyle, medial tibia, lateral tibia, and patella. Clinical MRI was graded with a modified WORMS score to assess the morphological analysis. 3-year follow up only included clinical evaluation and x-rays to study progression (n=8 completed so far).
Statistical methods
We used one-way ANOVA to investigate group differences in MRI parameters (MD, FA, T2) with radiographic severity after testing for normal distribution. We applied Bonferroni correction for multiple comparisons. We used Spearman correlation coefficient to study the association between MRI parameters and radiographic severity (KL score as well as overall and cartilage WORMS scores). Changes over time were tested with paired t-test. The ability of MRI to diagnose OA and severity was tested using binomial and multinomial logistic regression. All correlation were corrected by age and BMI.
Results
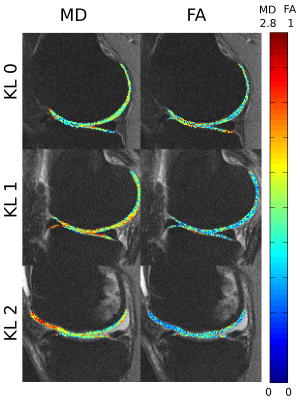
Figure 1 shows an example of the progression of diffusion parameters with KL grade. Correspondence between T2 and diffusion parameters is shown in Figure 2.Correlation with radiographic severity
We found a trend of increased MD and decreased FA with KL severity in the medial compartment (Fig. 3). This trend was only significant for the lateral compartment, specifically on the LT (p<0.05). MD increased was +15% and FA decreased was -16% between KL0 and KL2 subjects. T2 di not show any significant differences although T2 showed a trend towards increased values.
Correlation with WORMS score
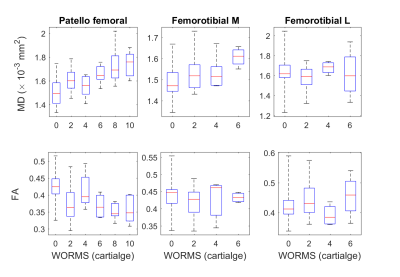
Correlations between MRI parameters and overall WORMS score was poor (ρ=0.15, p=0.15). We then investigated the correlation between MRI measurements and the cartilage WORMS score for cartilage plates (Fig.4). MD correlated with WORMS cartilage score and MD in the patellofemoral (ρ =0.50, p<0.001) and the lateral femorotibial compartments (ρ =0.36, p<0.02), and FA showed a negative correlation with WORMS (ρ =-0.33, p<0.02) in the lateral femorotibial compartment. Correlation of WORMS was not significant with T2.
Progression of MRI metrics
Over 1.5 years we observed significant differences in MD and FA in the lateral compartment. Average significant increase in MD was 5.3% in the MFC and 7.8% in the LT (p<0.05). FA was significantly decreased in the LT (-10.1%,p<0.05). Differences in T2 were not significant in any compartment. There were no significant changes in cartilage WORMS scores within the 1.5-y period.
Conclusions
We have been able to test the value of DTI as a diagnostic tool in an population in early stages of the disease and a likelihood of progression in a short period of time. DTI have proven as a sensitive biomarker to detect changes cross-sectionally. More importantly, changes in DTI parameters could be detected within a short time window of 1.5 y. Interestingly, MD changes seem to precede changes in FA, which may be interpreted as an indication that damage to the collage structure occurs secondary to proteoglycan loss. In summary, DTI has proven potential as a biomarker for cartilage integrity and potential for diagnosis and prognosis.Acknowledgements
This study has received funding from the (US) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institute of Health (NIH), Grant/Award Number R01AR067789. One of the authors received funding by "Fundacion Alfonso Martin Escudero".
References
1. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nature Reviews Rheumatology. 2014;10:437.
2. Hayashi D, Roemer FW, Guermazi A. Magnetic resonance imaging assessment of knee osteoarthritis: current and developing new concepts and techniques. Clin Exp Rheumatol. 2019;37 Suppl 120(5):88-95.
3. Filidoro L, Dietrich O, Weber J, et al. High-resolution diffusion tensor imaging of human patellar cartilage: feasibility and preliminary findings. Magn Reson Med. 2005;53(5):993-998.
4. de Visser SK, Bowden JC, Wentrup-Byrne E, et al. Anisotropy of collagen fibre alignment in bovine cartilage: comparison of polarised light microscopy and spatially resolved diffusion-tensor measurements. Osteoarthritis Cartilage. 2008;16(6):689-697.
5. Raya JG, Arnoldi AP, Weber DL, et al. Ultra-high field diffusion tensor imaging of articular cartilage correlated with histology and scanning electron microscopy. MAGMA. 2011;24(4):247-258.
6. Raya JG, Melkus G, Adam-Neumair S, et al. Change of diffusion tensor imaging parameters in articular cartilage with progressive proteoglycan extraction. Invest Radiol. 2011;46(6):401-409.
7. Duarte A, Ruiz A, Ferizi U, et al. Diffusion tensor imaging of articular cartilage using a navigated radial imaging spin-echo diffusion (RAISED) sequence. Eur Radiol. 2019;29(5):2598-2607.
Figures