0342
Multinuclear MRS at 7T uncovers exercise driven differences in skeletal muscle energy metabolism between young and seniors1High-field MR Center, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Institute of Experimental Endocrinology, Biomedical Research Center of Slovak Academy of Sciences, Bratislava, Slovakia, 3Department of Imaging Methods, Institute of Measurements Science, Slovak Academy of Sciences, Bratislava, Slovakia, 4Oxford Centre for Clinical Magnetic Resonance Research, BHF Centre of Research Excellence, University of Oxford, Oxford, United Kingdom, 5Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 6Institute of Pathophysiology, Faculty of Medicine, Commenius University, Bratislava, Slovakia, 7Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 8Division of Endocrinology and Metabolism, Department of Internal MedicineIII, Medical University of Vienna, Vienna, Austria
Synopsis
The aim of this study was to investigate effect on the demand driven ATP production and carnosine content in the aging muscle. We utilized dynamic and saturation transfer 31P- and 1H-MRS. The dynamic experiment included acquisition of baseline data during two minutes of rest, six minutes of plantar flexion exercise (3.5 minutes long FAST measurement was performed), and six minutes of recovery. We report excessive Pi-to-ATP flux and increase of PME concentration during exercise as well as lower muscle carnosine concentration leading to lower pH after exercise in seniors, which could be linked to deprived metabolic flexibility in this population.
INTRODUCTION
With the increasing average age of population, the associated musculoskeletal disorders, frailty and sarcopenia leading to a decline in physical function and increased risk for disability [1] are a growing concern. Skeletal muscle plays a crucial role in whole-body energy metabolism [2]. Thus, we utilized dynamic and saturation transfer (ST) phosphorus (31P) magnetic resonance spectroscopy (MRS), as well as proton (1H) MRS to investigate possible effect on the demand driven ATP production and carnosine content in the aging muscle.METHODS
The study population included 15 young (age 29±7 yrs; BMI 21.2±1.8 kg.m-2) and 19 elderly volunteers (age 65±6 yrs; BMI 26.7±4.3 kg.m-2). Experimental protocol consisted of acquisition of 1H-MRS and 31P-MRS at rest, and 31P-MRS acquired during 6 minutes of plantar flexion exercise and following 6 minutes of recovery. All measurements were performed on a whole-body 7T MR system (Siemens Healthineers, Erlangen, Germany). Participants were positioned supine with their right calf placed in a 28-channel knee coil (QED, Mayfield Village, OH, USA) for 1H-MRS carnosine signal acquisition first (water suppression, TR=9000 ms, TE=20 ms, NA=64 and excitation frequency centred at 8 ppm). Afterwards, participants were repositioned onto a MR-compatible ergometer (Trispect, Ergospect, Innsbruck, Austria) with the 31P/1H surface coil (10 cm, Rapid-Biomedical, Rimpar, Germany) positioned under their right calf for 31P-MRS. Depth-resolved in vivo spectroscopy (DRESS) was used for signal localization (15 mm thick slab through gastrocnemius muscle) in all static and dynamic 31P MRS experiments [3]. Pulse-acquire dynamic 31P MRS was performed in basal state (2 min), initial challenged state (2 min) and during the recovery (6 min). A four-angle saturation transfer (FAST) measurements taking 3.5 minutes were performed at rest as well as two minutes after the onset of exercise when a new steady state was reached [12]. The exercise was performed at a work load of approximately 30% of maximal voluntary contraction force with one flexion per TR (TR=2 s). All acquired spectra were fitted using time domain fitting routine AMARES in jMRUI [4].RESULTS
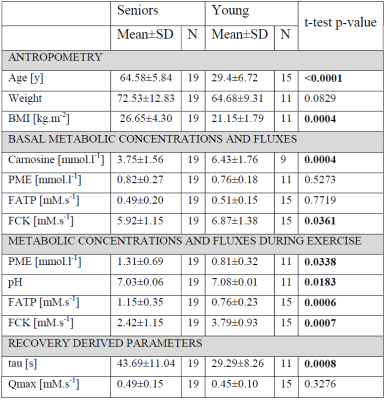
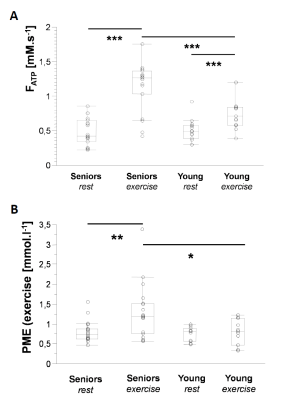
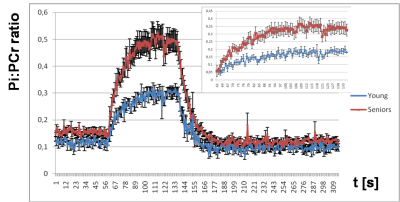
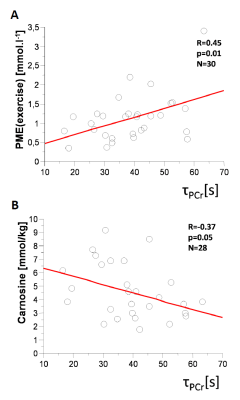
Elderly volunteers had significantly slower PCr recovery in comparison to the young (τPCr 43.7±11.0 s vs. 29.3±8.3 s; p=0.0008). Exercise caused a significant increment of Pi-to-ATP flux in both groups, i.e. from 0.49±0.20 mM.s-1 to 1.15±0.35 mM.s-1 (p =0.0001) in seniors and from 0.51±0.15 mM.s-1 to 0.76±0.23 mM.s-1 (p=0.002) in young (Figure 1A). There was also a intergroup difference in Pi-to-ATP flux during exercise (p=0.0006). Elderly volunteers had lower muscle carnosine concentration in basal state (3.75±1.56 mM vs. 6.43±1.76 mM; p=0.0004) and lower pH after exercise (7.03±0.06 vs. 7.08±0.01; p=0.02). We observed also significant increase in phosphor-monoesters (PME) during exercise in seniors, i.e. from 0.82±0.27 mM to 1.31±0.69 mM (p=0.006) Figure (1B). Measured Pi:PCr ratio attained during exercise was significantly higher in seniors than in young subjects, whereas Pi:PCr kinetic during recovery period was faster in young subjects compared to seniors (Figure 2). We observed positive correlation of tPCr with age (R=0.56; p=0.001), BMI (R=0.65; p=0.0001) and PME during exercise period (R=0.45; p=0.01) (Figure 3A). Besides, we found also a negative correlation of tPCr with the concentration of carnosine (R=-0.35; p=0.05) (Figure 3B). Moreover, we observed significantly lower pH after exercise (p=0.0183) in seniors and negative association of pH with τPCr (R=-0.37; p=0.0382. Detailed results are summarized in the Table 1.DISCUSSION
In our study, elderly subjects displayed longer PCr recovery time after exercise (τPCr) compared to young people what is in agreement with lower training status, lower physical activity and physical fitness in seniors [5-9]. This is also in good agreement with observation of lower Qmax in non-active subjects [10]. Higher Pi-to-ATP flux observed in seniors during the exercise may indicate the requirement of higher ATP production to cope with similar exercise load in comparison to young subjects. Measured Pi-to-ATP flux in young volunteers at rest, as well as increment during exercise is in agreement with previous reports [11, 12]. Exercise challenge was in seniors linked to strong increase of PME concentration (p=0.006), which on-the-other-hand did not reach significance (p=0.62) in young subjects. This observation can be explained by better capacity of young muscle to cope with work-load because increase in PME may be attributed to an accumulation of inosine monophosphate (IMP) [13] that resonate in the PME frequency range. Ageing is also associated with a loss of skeletal muscle mass, which was in biopsy studies shown to be connected with significant reduction in muscle carnosine content [14, 15]. Carnosine has positive effects on muscle strength and pH buffering properties. In accordance with these findings, we observed significantly lower muscle concentration of carnosine in seniors (p=0.0004). Furthermore, we have found a negative association of muscle carnosine concentration with age (R=0.57, p=0.001). Moreover, our observation of significantly lower pH after exercise in seniors together with negative association of pH with PCr recovery after exercise (τPCr) agrees with literature [16, 17] and also suggests decreased pH buffering ability due to lower levels of carnosine.CONCLUSION
Excessive Pi-to-ATP flux and increase of PME concentration during exercise in seniors result probably from the lower ability to cope with load compared to young subjects. Moreover, elderly volunteers had lower muscle carnosine concentration leading to lower pH after exercise.Acknowledgements
CTR and LV are funded by a Sir Henry Dale Fellowship from the Wellcome Trust [098436/Z/12/B. The support of the Slovak Grant Agencies VEGA [2/0001/17] and APVV [18–0029] is also gratefully acknowledged.References
1. Brady, A.O., C.R. Straight, and E.M. Evans, Body composition, muscle capacity, and physical function in older adults: an integrated conceptual model. J Aging Phys Act, 2014. 22(3): p. 441-52.
2. Frontera, W.R. and J. Ochala, Skeletal muscle: a brief review of structure and function. Calcif Tissue Int, 2015. 96(3): p. 183-95.
3. Valkovič, L., et al., Depth-resolved surface coil MRS (DRESS)-localized dynamic (31) P-MRS of the exercising human gastrocnemius muscle at 7 T. NMR Biomed, 2014. 27(11): p. 1346-52.
4. Vanhamme, L., A. van den Boogaart, and S. Van Huffel, Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson, 1997. 129(1): p. 35-43.
5. Boulton, E.R., M. Horne, and C. Todd, Multiple influences on participating in physical activity in older age: Developing a social ecological approach. Health Expect, 2018. 21(1): p. 239-248.
6. Speakman, J.R. and K.R. Westerterp, Associations between energy demands, physical activity, and body composition in adult humans between 18 and 96 y of age. Am J Clin Nutr, 2010. 92(4): p. 826-34.
7. Ayabe, M., et al., Objectively measured age-related changes in the intensity distribution of daily physical activity in adults. J Phys Act Health, 2009. 6(4): p. 419-25.
8. Hawkins, M.S., et al., Objectively measured physical activity of USA adults by sex, age, and racial/ethnic groups: a cross-sectional study. Int J Behav Nutr Phys Act, 2009. 6: p. 31.
9. Buchman, A.S., et al., Total daily activity declines more rapidly with increasing age in older adults. Arch Gerontol Geriatr, 2014. 58(1): p. 74-9.
10. Valkovic, L., et al., Skeletal muscle alkaline Pi pool is decreased in overweight-to-obese sedentary subjects and relates to mitochondrial capacity and phosphodiester content. Sci Rep, 2016. 6: p. 20087.
11. Sleigh, A., et al., 31P magnetization transfer measurements of Pi-->ATP flux in exercising human muscle. J Appl Physiol (1985), 2016. 120(6): p. 649-56.
12. Tusek Jelenc, M., et al., Feasibility and repeatability of localized (31) P-MRS four-angle saturation transfer (FAST) of the human gastrocnemius muscle using a surface coil at 7 T. NMR Biomed, 2016. 29(1): p. 57-65.
13. Soussi, B., et al., Dynamics of skeletal muscle energetics during ischemia and reperfusion assessed by in vivo 31P NMR. NMR Biomed, 1990. 3(2): p. 71-7.
14. Stuerenburg, H.J. and K. Kunze, Concentrations of free carnosine (a putative membrane-protective antioxidant) in human muscle biopsies and rat muscles. Arch Gerontol Geriatr, 1999. 29(2): p. 107-13.
15. Tallon, M.J., et al., Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology, 2007. 8(2): p. 129-37.
16. Iotti, S., et al., In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. The role of pH in the evaluation of phosphocreatine and inorganic phosphate recoveries from exercise. NMR Biomed, 1993. 6(4): p. 248-53.
17. Layec, G., et al., Effects of exercise-induced intracellular acidosis on the phosphocreatine recovery kinetics: a 31P MRS study in three muscle groups in humans. NMR Biomed, 2013. 26(11): p. 1403-11.
18. Kreukels, B.P.C., et al., ERP amplitude and latency in breast cancer survivors treated with adjuvant chemotherapy. Clin Neurophysiol, 2008. 119(3): p. 533-541.
Figures