0337
Radiomic Analysis Based on Diverse Volumetric Interests Predicts Microvascular Invasion in Solitary Hepatocellular Carcinoma1Department of Radiology, Shanghai Institute of Medical Imaging, Zhongshan Hospital of Fudan University, Shanghai, China, 2Shanghai United Imaging Intelligence Co., Ltd, Shanghai, China
Synopsis
This study aims to construct a preoperative MVI prediction model in solitary HCC derived from gadoxetic acid-enhanced magnetic resonance imaging, and to further investigate its latent association with clinical indexes, imaging features and radiomics signatures based on diverse sequences and volumetric interests (VOIs) of tumor. The conclusion indicated that serum α-fetoprotein, total bilirubin, higher value of tumor margin smoothness (prefer to non-smooth margin), non-intact capsule enhancement and peritumoral enhancement are independent and significant predictors for MVI, and the final nomogram incorporating clinical, imaging and the optimal radiomics model based on VOI_entire_5mm_10mm_liver achieves satisfactory prediction for MVI in solitary HCC.
Introduction
Microvascular invasion (MVI) as an independent and significant prognostic factor for hepatocellular carcinoma (HCC) [1], is only visible on microscopy [2,3],which limits preoperative strategies of wide resection margin in MVI positive patients [4]. Radiomics as a novel and noninvasive technique can associate preoperative imaging signatures with postoperative tumor pathophysiology, which is conducive to the implementation of preoperative strategy.Methods
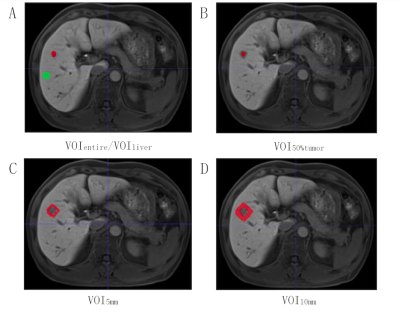
Three hundred and fifty-six consecutive patients with pathologically confirmed solitary HCC (training cohort: n=250; validation cohort: n=106) who underwent preoperative gadoxetic acid-enhanced magnetic resonance (MR) imaging were enrolled. Univariate logistic regression analysis was applied to identify the significant and independent clinical and primary imaging features associated with MVI, and then construct the corresponding models using random forest (RF) and logistic regression (LR) analysis. Reducing dimensionality by univariate Feature Selection (UFS) and least absolute shrinkage and selection operator (LASSO), radiomics models fused diverse volumetric interests (VOIs) and multi-sequence fusion signatures using RF and LR, the optimal of which was then incorporated clinical and imaging models into the final MVI predictive nomogram.Results
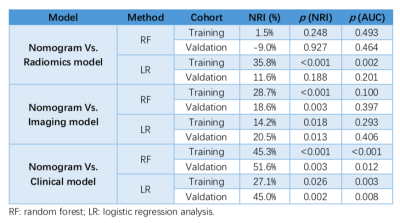
Higher AFP value (AFP: 20-400 ng/mL, p=0.01; AFP >400 ng/mL, p=0.024), TBIL >20.4 umol/L (p=0.011), higher level of tumor margin smoothness (prefer to non-smooth margin, p=0.015), non-intact capsule enhancement (p<0.001) and peritumoral enhancement (p=0.01) were independent and significant predictors for MVI in solitary HCC. In validation cohort, the AUC of clinical model was 0.725 using RF and 0.668 using LR, and was 0.875 using RF and 0.791 using LR in imaging model, respectively. The best multi-sequence model was the fusion of “Venous+HBP+Arterial+DWI” based on the VOI of entire tumor, peritumoral 5mm, peritumoral 10mm and normal hepatic tissues (VOI_entire_5mm_10mm_liver), with AUC of 0.918 using RF and 0.809 using LR in validation cohort. The AUC of final MVI prediction model was 1.0 using RF and 0.948 using LR in training cohort, and was 0.926 using RF and 0.883 using LR in validation cohort. Besides, the NRI of MVI prediction model statistically exceed clinical and imaging models both in training (range: 14.2%-45.3%) and validation cohort (range: 18.6%-51.6%) no matter using RF or LR. In addition, the NRI of MVI prediction model excelled radiomics model using LR (35.8% in training cohort and 11.6% in valdation cohort), but was slight or negative improvement using RF without statistical differences. Visualized by the nomogram, the final predictive model fit well with the MVI status in calibration curves, and decision curve analysis further confirmed the clinical significance of the nomogram.Discussion
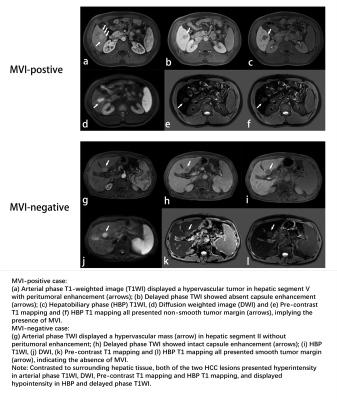
Peritumoral tissue is the first and most frequently vulnerable to MVI, vessels of which further serves as the main hematogenous dissemination pathway of portal vein tumor thrombosis, intrahepatic and extrahepatic metastasis[5]. Consequently, the imaging features of peritumoral tissue such as tumor margin smoothness, capsule enhancement and peritumoral enhancement, as well as radiomics signatures based on diverse VOIs, might be more representative of MVI involvement than inside the tumor itself.Conclusions
The nomogram incorporating clinical, primary imaging features and the optimal radiomics signatures based on the VOI_entire_5mm_10mm_liver derived from gadoxetic acid-enhanced MRI obtained satisfactory prediction for preoperative MVI status in solitary HCC patients.Acknowledgements
No acknowledgement found.References
[1] LEE S, KIM S H, LEE J E, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma [J]. J Hepatol, 2017, 67(3): 526-534.
[2] CONG W M, BU H, CHEN J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update [J]. World J Gastroenterol, 2016, 22(42): 9279-9287.
[3] LEI Z, LI J, WU D, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria [J]. JAMA Surg, 2016, 151(4): 356-363.
[4] HWANG S, LEE Y J, KIM K H, et al. The Impact of Tumor Size on Long-Term Survival Outcomes After Resection of Solitary Hepatocellular Carcinoma: Single-Institution Experience with 2558 Patients [J]. J Gastrointest Surg, 2015, 19(7): 1281-1290.
[5] HU H T, SHEN S L, WANG Z, et al. Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis [J]. Abdom Radiol (NY), 2018, 43(12): 3324-3330.