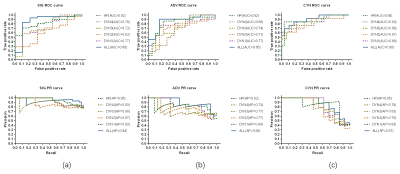0334
Imaging-based Hepatic fibrosis staging of patients with hepatitis B: a radiomics analysis study on Gd-EOB-DTPA-enhanced MRI1Fudan University, Shanghai, China, 2ShanghaiPublic Health Clinical Center, Shanghai, China, 3Philips Healthcare, Shanghai, China, 4Human Phenome Institute, Fudan University, Shanghai, China, 5Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
Synopsis
This study proposed an automatic hepatic fibrosis staging model based on transfer learning segmentation and radiomics analysis for hepatitis B patients. The automatic liver ROI extarction Time dimension features of multi DCE phases were included in feature set which played an important role in the classification. The proposed model exhibited a superior performance in significant fibrosis, advanced fibrosis and cirrhosis classification.
INTRODUCTION
Hepatic fibrosis as the common pathological process of variety chronic liver diseases, which is reflected the liver damage response to various causes1. The treatment for chronic liver diseases is highly dependent on the evaluation of fibrosis degrees in clinical diagnosis. At present, liver biopsy is still the gold standard for diagnosing liver fibrosis. However, this inspection is not accepted by most patients, especially for patients with low-grade fibrosis and relatively stable diseases due to the intrusiveness of the test2. The primary objective of this study was to phase hepatic fibrosis automatically with radiomics analysis.METHODS
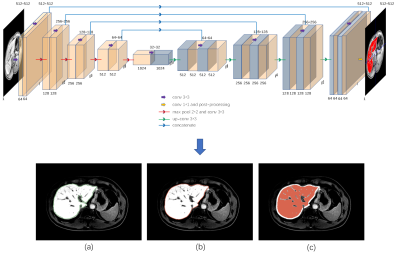
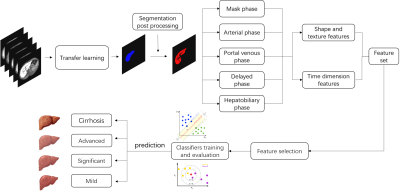
132 hepatitis B patients were recruited into the subject group for Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) enhanced magnetic resonance (MR) scans, which included four Scheuer-Ludwig scoring (S)3 types: 30,28, 32, 42 patients with fibrosis stages S0, S1, S2, S3, and S4, respectively; mean patient age, 45.80±13.17 years; 93 males and 39 females. All patients have five DCE phases: mask phase, arterial phase, portal venous phase, delayed phase and hepatobiliary phase (HP). The proposed fully automated liver fibrosis staging model is performed as follows. (a) Transfer learning was applied for automatic whole-liver segmentation, where large portal veins were excluded by some post processing algorithms which based on the connecting component. The processed areas was regarded as the region of interest (ROI). The neural network architecture is a classical U-net based model, which is illustrated in the top of Figure 1; (b) A large number of features including time dimension features (a total of 1288 features including 14 common shape features, 455 phase texture features, 364 time curve features and 455 time difference features) were extracted in multi Dynamic Contrast Enhanced (DCE) phases; (c) the least absolute shrinkage and selection operator (LASSO)4 logistic regression algorithm was adopted to filter essential elements to form a feature subset; (d) Various classifiers were trained based on the feature subset and evaluated in 5-fold cross-validation to select the optimal classifier for prediction in the external test set. In addition, the classification performance was obtained by the Receiver operating characteristic (ROC), the Precision-Recall (PR) curve, and the F1 score analysis. The overall framework is shown in Figure 2.RESULTS
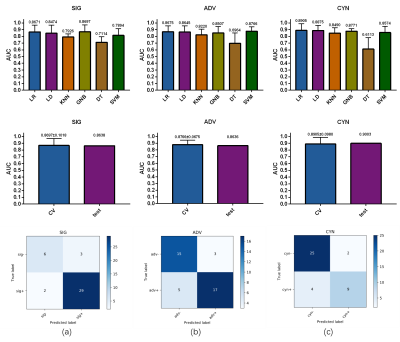
In this study, the quadratic classification issue was turned into three binary classification tasks: significant fibrosis (S2-4 vs S1), advanced fibrosis (S3-4 vs S1-2) and cirrhosis (S4 vs S1-3) classification. 40 of 132 cases were randomly withdrawn to formulate the external test set (with the original distribution of positive and negative cases), with the remaining 92 were regarded as training set for each classification task. The results show that the classification performance in external test set achieves accuracy of 0.8750, 0.8000, 0.8500, Area Under Curve (AUC) value of 0.8638, 0.8636, 0.9003 average precision score (AP) of 0.9375, 0.8956, 0.8658 and F1 score of 0.9206, 0.8095, 0.7500 for significant fibrosis, advanced fibrosis and cirrhosis, respectively. Otherwise, by comparing the results with single phase-based model, multi DCE phases feature extraction model is more dominant. The performance of the studied six mainstream classifies in 5-fold cross-validation, the AUC value comparison of the selected optimal classifier in cross-validation and external test, and the confusion matrix in external test set are illustrated in Figure 3.The ROC curves and PR curves are demonstrated in Figure 4, the detailed performance parameters see Table 1.DISCUSSION
The results indicated that the proposed model is adequate for significant fibrosis, advanced fibrosis and cirrhosis classification as the accuracy exceeds 0.80 and AUC exceeds 0.86 for all the tasks. Multi DCE phases feature extraction model has more superior performance than models only considering one phase, which suggests the importance of time dimension features in hepatic fibrosis staging. Actually, in the final feature subset which contains only five features, four features belonged to time dimension for significant and advanced fibrosis, while two time dimensions for cirrhosis classification. The study found that HP was critical to the results of hepatic fibrosis staging as well, which outperformed other individual phases in classification. This is also consistent with previous research results.CONCLUSION
This study proposed an automatic hepatic fibrosis staging method based on radiomics analysis in multiple Gd-EOB-DTPA-enhanced DCE phases. Automatic extraction of liver ROI based on transfer learning could save doctors’ time and energy to a large extent. More valuable features associated with liver fibrosis grading could be obtained through a larger feature set combining multi DCE phases texture features and time dimension features, results in a better classification performance. In general, the proposed automatic staging model can help doctors quickly and accurately assess the degree of liver fibrosis, and choose the appreciate treatment plan in clinical diagnosis.Acknowledgements
Acknowledge: This work was supported by Shanghai Municipal Science and Technology Major Project (No.2017SHZDZX01), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZJLab, Shanghai Natural Science Foundation (No. 17ZR1401600) and the National Natural Science Foundation of China (No. 81971583).References
1. Bataller R, Brenner DA. Liver fibrosis. The Journal of clinical investigation 2005;115:209-218.
2. Chang PE, Goh GB, Ngu JH, Tan HK, Tan CK. Clinical applications, limitations and future role of transient elastography in the management of liver disease. World journal of gastrointestinal pharmacology and therapeutics 2016;7:91-106.
3. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. Journal of hepatology 1991;13:372-374.
4. Tibshirani R. Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996;58:267-288.
Figures