0332
A Deep Transfer Learning Model for Liver Stiffness Classification using Clinical and T2-Weighted MRI Data1The Perinatal Institute and Section of Neonatology, Perinatal and Pulmonary Biology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Imaging Research Center, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 3Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 4Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 5Department of Radiology, University of Cincinnati College of Medicine, Cincinnati, OH, United States
Synopsis
Detection and monitoring of chronic liver diseases is typically assessed using a combination of clinical history, physical examination, laboratory testing, biopsy with histopathologic assessment, and imaging. The aim of this study is to develop a deep transfer learning model (DeepLiverNet) to categorically classify the severity of liver stiffening (no/mild vs. moderate/severe) using both anatomic T2-weighted MR images and clinical data. The DeepLiverNet model achieved accuracies of 88.0% and 80.0% on the risk stratification of liver stiffness in internal and external validation datasets, respectively. This demonstrates that a deep learning model may provide a means for stratifying liver stiffness without elastography.
INTRODUCTION
Chronic liver diseases are a common source of morbidity and mortality in both children and adults in the United States and around the world. 1,2 Detection and progression of such liver diseases is typically assessed using a combination of clinical history, physical examination, laboratory testing, biopsy with histopathologic assessment, and imaging. 3 MR elastography (MRE), a non-invasive method of assessing liver stiffness, uses an active-passive driver system (with the passive paddle placed over the right upper quadrant of the abdomen at the level of the costal margin) to create transverse (shear) waves in the liver. 4 Although MRE may obviate the need for liver biopsy in some patients and allows more frequent longitudinal assessment of liver health, it has associated drawbacks related to additional patient time in the scanner, patient discomfort, and added costs (e.g., infrastructure and patient charge-related). 5 In the current project, we aim to develop a deep learning approach to categorically classify liver stiffness-determined by MRE-using both T2-weighted imaging and clinical data from pediatric and young adult patients.METHODS
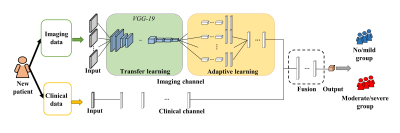
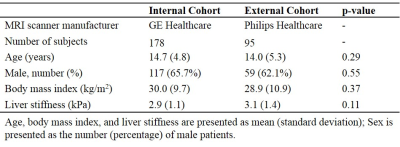
In this retrospective study, we collected 178 MRE examinations from a GE scanner for model development and internal validation and 95 MRE examinations from a Philips scanner for external validation. For each subject, the mean liver stiffness value in kPa (shear modulus) and 27 clinical features were retrieved from the electronic health record (Epic Systems Corporation; Verona, WI). Based on pre-defined liver stiffness cutoff, 6 patients were divided into two groups (<3 kPa=no/mild vs. ≥3 kPa=moderate/severe liver stiffening). Axial two-dimensional T2-weighted fast spin-echo fat-suppressed images were extracted from our clinical Picture Archiving and Communicating System.Our deep model contains two separate input channels for imaging and clinical data, respectively (Figure 1). The imaging channel is comprised of an image input layer, a transfer learning block, and an adaptive learning block. First, the image input layer contains S parallel input sub-channels, taking S individual slices of fixed-size axial T2-weighted MR images. Next, to extract liver image features, we designed a transfer learning block by reusing the weights of a VGG-19 model 7 (from 1st to 21st layers) that was trained based on ~1.2 million color images from ImageNet database. 8 Then, we designed an adaptive learning block that contains S parallel sub-channels (two convolutional layers with [8, 16] neurons and 3×3 filters, and a fully-connected layer with 8 neurons) corresponding to the input sub-channels for learning the individual latent features of S liver slices, respectively. In the end, those sub-channels in the adaptive learning block are integrated by a fully-connected layer with 8 neurons. In the current study, we used four axial T2-weighted images of the liver from the same anatomic levels as MRE images (i.e., S=4). For the clinical channel, a fully-connected layer with 8 neurons is directly applied. After the feature extraction, a fusion block (8 neurons) is applied to integrate the latent features from both imaging and clinical data. A two-way softmax classifier was utilized to classify the severity of liver stiffness.
Considering the imbalanced subject ratio (e.g., <3 vs. ≥3 kPa = ~2:1 in the current study), a rotation and shift-based data augmentation scheme 9 is used to balance the subjects between two groups. The diagnostic performance of the model is assessed with the metrics of accuracy, sensitivity, specificity, and area under the receiver operating characteristic curve (AuROC). We used 10-fold cross-validation in the internal validation with the internal cohort. In the external validation, we trained the model with the internal cohort and tested the model with an external cohort.
RESULTS
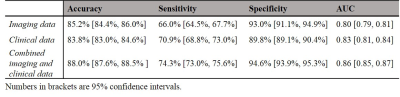
Demographics of two cohorts were listed in Table 1.Internal Validation. We first set to determine the performance of DeepLiverNet using only non-stiffness T2-weighted imaging data, including liver volume and chemical shift-encoded fat fraction. As shown in Table 2, the DeepLiverNet was able to correctly classify patients with regard to categorical MRE liver stiffness with an AuROC of 0.80. Using only clinical data, the model classified patients with an AuROC of 0.83, achieving a significantly greater AuROC (p=0.003) compared to the one using only imaging data. The DeepLiverNet combining both T2-weighted MR imaging and clinical data was able to correctly classify patients with an AuROC of 0.86. This was significantly greater than imaging data alone (p<0.0001) or clinical data alone (p<0.0001).
External Validation. The internally-validated DeepLiverNet using both clinical and imaging features achieved an accuracy of 80.0%, with a sensitivity of 61.1%, a specificity of 91.5%, an AuROC of 0.77 on independent external subjects.
DISCUSSIONS and CONCLUSIONS
In this work, we applied both transfer learning and data augmentation strategies to avoid deep learning model overfitting. Our model demonstrated good generalizability when externally validated. Our proposed deep learning model that incorporated clinical features and T2-weighted MR images demonstrated a means of classifying patients into normal/minimally elevated versus moderately/severely elevated liver stiffness with an accuracy up to 88%. Further studies are needed to continue to refine the model as well as validate it in other patient groups, including older adults and cohorts with very specific liver diseases (e.g., sclerosing cholangitis, viral hepatitis, non-alcoholic fatty liver disease).Acknowledgements
We highly appreciated the internal research grant of the Department of Radiology, Cincinnati Children's Hospital Medical Center.References
1. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
2. Lavanchy D. The global burden of hepatitis C. Liver international. 2009;29:74-81.
3. Tapper EB, Lok AS-F. Use of liver imaging and biopsy in clinical practice. New England Journal of Medicine. 2017;377(8):756-768.
4. Trout AT, Sheridan RM, Serai SD, et al. Diagnostic performance of MR elastography for liver fibrosis in children and young adults with a spectrum of liver diseases. Radiology. 2018;287(3):824-832. 5. Wang M, Byram B, Palmeri M, Rouze N, Nightingale K. Imaging transverse isotropic properties of muscle by monitoring acoustic radiation force induced shear waves using a 2-D matrix ultrasound array. IEEE transactions on medical imaging. 2013;32(9):1671-1684.
6. He L, Li H, Dudley JA, et al. Machine Learning Prediction of Liver Stiffness Using Clinical and T2-Weighted MRI Radiomic Data. American Journal of Roentgenology. 2019:1-10.
7. Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv preprint arXiv:14091556. 2014.
8. Deng J, Dong W, Socher R, Li L-J, Li K, Fei-Fei L. Imagenet: A large-scale hierarchical image database. IEEE Conference on Computer Vision and Pattern Recognition. 2009.
9. Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. Advances in Neural Information Processing Systems. 2012.
Figures