0331
Preoperative prediction of HCC with highly aggressive characteristics using quantitative parameters derived from hepatobiliary phase1West China Hospital, Sichuan University, Chengdu, China
Synopsis
Hepatocellular carcinoma (HCC) with highly aggressive characteristics is usually actively proliferated and easily relapse, thereby requiring adjuvant therapies before surgery, like preoperative TACE, to improve the patients’ prognosis. Ki-67 labeling index (LI) was reported to be highly correlated with aggressive propensity of HCC, and thus could affect the treatment response of the tumor and prognosis directly. Although most of HCC presented hypointensity on hepatobiliary phase (HBP), the absolute signal intensity and relative contrast enhancement ratio are not the same. In this study, we prospectively investigate the usefulness of HBP quantitative parameters for preoperative prediction of aggressiveness in HCC patients.
Purpose
To prospectively determine whether the quantitative imaging parameters derived from hepatobiliary phase (HBP) can be used for the preoperative prediction of hepatocellular carcinoma (HCC) with highly aggressive characteristics.Materials and Methods
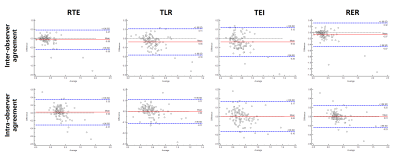
One hundred and three patients with surgical-proven HCC were included from July 2015 to June 2018. All MRI examinations were performed on a 3.0T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with an 18-channel phased torso coil. T1-weighted volume interpolated breath hold (VIBE) gradient recall echo sequence was used for the acquisition of pre-enhancement images, dynamic contrast enhanced phases and 20 mins HBP. Signal intensity (SI) was measured by two independent reviewers. For liver parenchyma, three circle region of interest (ROIs) with 3cm2 area were respectively placed on the left lobe, right lobe anterior and posterior segments, avoiding major vessels, tumor, and bile ducts. For HCC lesion, three freehand ROIs were draw along the tumor margin at or next the level of the largest diameter of the tumor, excluding vessels and necrotic areas (Figure 1). The average SI of liver parenchyma and tumor was recorded as L0 and T0 based on PRE; and as L20 and T20 based on HBP, accordingly. Quantitative parameters including relative tumor enhancement [RTE, (T20-T0)/T0], tumor to liver contrast ratio (TLR, T20/L20), tumor enhancement index [TEI, (T20/L20)/(T0/L0)] and relative enhancement ratio [RER, (T20-T0)/(L20-L0)] were calculated. The aggressive characteristics of HCC was identified by using Ki-67 labeling index (LI) and classified into low aggressive (Ki-67 LI ≤ 10%) and high aggressive (Ki-67 LI > 10%) groups. Difference of quantitative parameters between two groups were assessed and the correlation between quantitative parameters and Ki-67 LI was explored. Receiver operating characteristic analyses was used to evaluate the predictive performance of quantitative parameters. To evaluate the inter-observer and intra-observer agreement of quantitative parameters, intraclass correlation coefficient (ICC) and the Bland-Altman plots were illustrated.Results
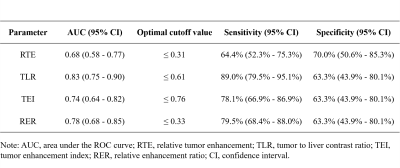
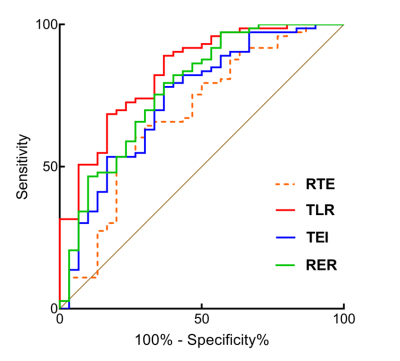
There was no significant difference in age, sex ratio, tumor size and laboratory results between low and high aggressive groups in HCC patients (p > 0.05). The values of RTE, TLR, TEI and RER were significantly lower in high aggressive group than low aggressive group (p < 0.05) (Figure 2) and negative correlations were obtained between these quantitative parameters and Ki-67 LI (r ranges from -0.41 to -0.22, p < 0.05). TLR demonstrated the highest predictive performance with the area under curve (AUC) of 0.83 (95% confidence interval: 0.75-0.90), sensitivity of 89.0% and specificity of 63.3% (Figure 3), and subsequent with RER, TEI, and RTE with AUC of 0.78, 0.74 and 0.68 (Figure 4). Good to excellent inter-observer agreement (ICC ranges from 0.85-0.89) and intra-observer agreement (ICC ranges from 0.83-0.92) were found in the quantitative parameters (Figure 5).Discussion
Ki-67 LI has been reported as an independent prognostic indicator of HCC in previous studies1-2. In current study, we found that HCCs in high aggressive group showed significantly lower RTE, TLR, TEI and RER, which agreed with previous studies that HCC with lower relative tumoral SI on HBP had more aggressive biological behavior, such as advanced tumor grades and shorter progression-free survival (PFS) rate. Jin et al. found that poorly differentiated HCC could be preoperatively identified by TEI, which was also reported to be one of the independent predictive indicators of HCC with E-S grade IV3. Additionally, RER was found to be negatively correlated with HCC histological grades (r = -0.775, p < 0.001). Moreover, HCC patients with lower preoperative TLR showed higher tumor grades and shorter PFS rate after hepatic arterial infusion chemotherapy4. The reason why aggressive HCC tend to demonstrate lower relative tumoral SI probably is that normal hepatocytes gradually turns into uncontrolled and actively proliferated malignant tumor cells with higher Ki-67 LI during multistep hepatocarcinogenesis, while at the same time, the expression of OATP usually decreased, hence resulting in less uptake of Gd-EOB-DTPA.Conclusion
TLR showed the highest predictive ability in HCC with high aggressiveness. Thus, our results demonstrated that quantitative parameters based on the SI measurement of HBP could preoperatively and noninvasively predict the aggressive characteristics of HCC.Acknowledgements
None.References
1. Cao Y, Ke R, Wang S, Zhu X, Chen J, Huang C, Jiang Y, Lv L. DNA topoisomerase IIα and Ki67 are prognostic factors in patients with hepatocellular carcinoma. Oncology letters 2017;13:4109-16.
2. Li H-H, Qi L-N, Ma L, Chen Z-S, Xiang B-D, Li L-Q. Effect of KI-67 positive cellular index on prognosis after hepatectomy in Barcelona Clinic Liver Cancer stage A and B hepatocellular carcinoma with microvascular invasion. OncoTargets and therapy 2018;11:4747-54
3. Jin YJ, Cho SG, Lee KY, Kim JM, Lee JW. Association between relative liver enhancement on gadoxetic acid enhanced magnetic resonance images and histologic grade of hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e7580.
4. Fujita N, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Takayama Y, Okamoto D, Morita K, Shirabe K, Koto K, Kubo Y, Oda Y, Honda H. Significance of the Signal Intensity of Gadoxetic Acid-enhanced MR Imaging for Predicting the Efficacy of Hepatic Arterial Infusion Chemotherapy in Hepatocellular Carcinoma. Magn Reson Med Sci 2016;15:111-20.
Figures