0327
Modifying LI-RADS on gadoxetate disodium-enhanced MRI in a prospective cohort: toward improving simplicity and sensitivity1Department of Radiology, West China Hospital, Sichuan University, Chengdu, China, 2Department of Radiology, Duke University Medical Center, Durham, NC, United States
Synopsis
We aimed to develop a modified Liver Imaging Reporting and Data System (mLI-RADS) with comparisons against original LI-RADS version 2018 (v2018) for diagnosing hepatocellular carcinoma (HCC) on gadoxetate disodium-enhanced magnetic resonance imaging (EOB-MRI). 1002 hepatic observations in 272 consecutive at-risk patients were prospectively included. Ancillary features were assessed based on inter-rater agreement, prevalence, diagnostic accuracy, and added value to the major features. Compared with the original LI-RADS v2018, mLI-RADS demonstrated superior simplicity, sensitivity and accuracy without substantial loss of specificity; hence should be the preferred diagnostic criteria for HCC in high-risk patients on EOB-MRI.
Introduction
Liver Imaging Reporting and Data System (LI-RADS) was developed to standardize lexicons and categorizations for liver observations in patients at risk for hepatocellular carcinoma (HCC) and is widely accepted as a reliable diagnostic scheme with almost perfect specificity1-3. However, major flaws of LI-RADS include limited diagnostic sensitivity4-7, unclear utility of the overwhelmingly large number of ancillary features (AFs)4,5, and suboptimal compatibility with gadoxetate disodium enhanced magnetic resonance imaging (EOB-MRI)4,5,8,9. Prior studies have reported promising results of developing LI-RADS-based systems to diagnose HCC with EOB-MRI10-15, but most were retrospective in design12-15 with the utility of AFs remaining unclear.Therefore, we aimed to develop a modified LI-RADS (mLI-RADS) using EOB-MRI in at-risk patients to address the above pitfalls related to limited sensitivity and AFs and to compare its performance with the current LI-RADS version 2018 (v2018).
Methods
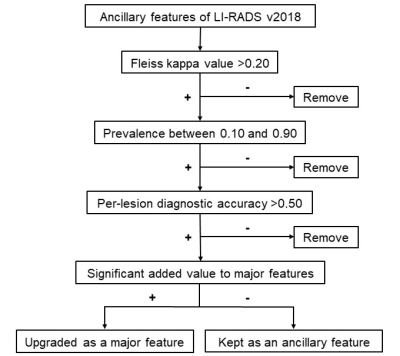
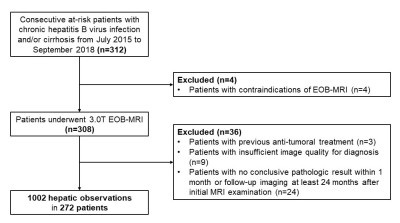
Our Institutional Review Board approved this single-center study. The acquisition of informed consent was waived because we retrospectively used data from a prospective clinical cohort [Clinical trial registration No: ChiCTR1900026668]. Between July 2015 and September 2019, we enrolled consecutive at-risk patients who underwent 3.0T EOB-MRI at our tertiary care hospital. Three blinded abdominal radiologists independently reviewed all MR images for major and ancillary features and assigned LI-RADS categories to each observation. Composite reference standards of either histopathologic or imaging follow-up were used for HCC diagnosis.To generate mLI-RADS, we selected and modified AFs based on inter-rater agreement (Fleiss’ kappa), prevalence, diagnostic accuracy and added value to the major features according to a stepwise algorithm (Figure 1). Binary logistic regression analyses were used to determine the added values of AFs to major features, and diagnostic measures including sensitivities, specificities, and accuracies were computed for individual imaging features, LI-RADS v2018 category, and mLI-RADS category. McNemar’s test was used to compare sensitivities and specificities pairwise.
Results
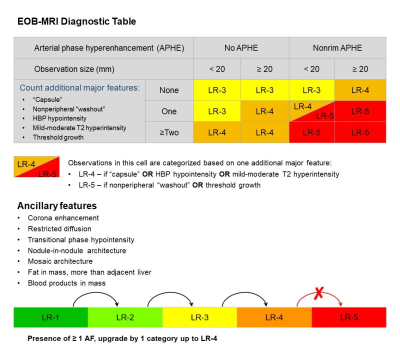
272 consecutive patients (220 males, 80.9%) were included (Figure 2), 87.5% (238/272) of whom chronic hepatitis B virus (HBV) carriers. Among the 1002 included hepatic observations (median size: 12 mm, range: 3-149 mm, 549 HCCs), 408 (40.7%), 272 (27.1%) and 322 (32.1%) were < 10 mm, between 10-19 mm and ≥ 20 mm, respectively. Based on the feature selection algorithm (Figure 1), hepatobiliary phase hypointensity and mild-moderate T2 hyperintensity were selected to become major features; enhancing “capsule” and non-enhancing “capsule” were integrated to form a new major feature—“capsule”; observation size ranges <10 mm and 10-19 mm were combined as a new size category < 20 mm; and only 7 AFs were retained while 14 AFs were removed completely (Figure 3).Based on LI-RADS v2018, the respective frequencies of HCC in LR-1, LR-2, LR-3, LR-4, LR-5, LR-TIV and LR-M categories were 0.0%, 0.0%, 25.3%, 88.5%, 94.3%, 73.3% and 41.8% by consensus; based on mLI-RADS, these were 0.0%, 0.0%, 21.4%, 75.8%, 92.5%, 73.3% and 41.8%. The per-lesion sensitivity, specificity and accuracy of LR-5 for LI-RADS v2018 were 45.5%, 96.7% and 68.7%, and for mLI-RADS were 60.8%, 94.0% and 75.8%. The sensitivity of mLI-RADS was significantly higher than LI-RADS v2018 (p<0.001), while the specificities were not significantly different (p=0.057). AFs altered the final categories in 7.6% (76/1002) observations by LI-RADS v2018, but only in 0.6% (6/1002) observations by mLI-RADS. The weighted kappa value for LI-RADS v2018 categories decided by major features alone and by major along with ancillary features was 0.74 and 0.73, respectively. These measures were 0.74 and 0.74 for LI-RADS, respectively.
Discussion
In this relatively large and balanced prospective cohort, the sensitivity (60.8% vs 45.5%) and overall accuracy (75.8% vs 68.7%) of mLI-RADS were superior compared with LI-RADS v2018, while the specificities of mLI-RADS (94.0%) and LI-RADS V2018 (96.7%) were similar and both excellent. In addition, the mLI-RADS demonstrated improved simplicity with only 7 major features and 7 AFs included over LI-RADS v2018 which consisted of 5 major features and 21 AFs, while maintaining comparable inter-rater agreement. Interestingly, the impact of AFs was also lower on the final categories using mLI-RADS than LI-RADS v2018. Since AFs are currently considered optional and cannot upgrade observations from LR-4 to LR-5, the whole set of AFs may be removed from the diagnostic system to further improve simplicity.Conclusion
Using EOB-MRI in high-risk patients, mLI-RADS demonstrated superior simplicity, sensitivity and overall accuracy for HCC than v2018 LI-RADS without loss of specificity. Thus, it may be considered the preferred diagnostic criteria over the original LI-RADS on EOB-MRI.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81771797) and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18008).References
1. American College of Radiology. CT/MRI LI-RADS version 2018. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed November 1, 2019.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
3. Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289(3):816-830.
4. Elsayes KM, Fowler KJ, Chernyak V, et al. User and system pitfalls in liver imaging with LI-RADS. J Magn Reson Imaging. 2019.
5. Kim YY, Choi JY2, Sirlin CB, et al. Pitfalls and problems to be solved in the diagnostic CT/MRI Liver Imaging Reporting and Data System (LI-RADS). Eur Radiol. 2019;29(3):1124-1132.
6. Kim DH, Choi SH, Park SH, et al. Meta-analysis of the accuracy of Liver Imaging Reporting and Data System category 4 or 5 for diagnosing hepatocellular carcinoma. Gut. 2019;68(9):1719-1721.
7. Kim YY, Kim MJ, Kim EH, et al. Hepatocellular Carcinoma versus Other Hepatic Malignancy in Cirrhosis: Performance of LI-RADS Version 2018. Radiology. 2019;291(1):72-80.
8. Chernyak V, Fowler KJ, Heiken JP, Sirlin CB. Use of gadoxetate disodium in patients with chronic liver disease and its implications for liver imaging reporting and data system (LI-RADS). J Magn Reson Imaging. 2019;49(5):1236-1252.
9. Kim DH, Choi SH, Byun JH, et al. Arterial subtraction images of gadoxetate-enhanced MRI improve diagnosis of early-stage hepatocellular carcinoma. J Hepatol. 2019;71(3):534-542.
10. Choi SH, Byun JH, Lim YS, et al. Diagnostic criteria for hepatocellular carcinoma ⩽3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol. 2016;64(5):1099-1107.
11. Renzulli M, Biselli M, Brocchi S, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut. 2018;67(9):1674-1682.
12. Kim DH, Choi SH, Kim SY, et al. Gadoxetic Acid-enhanced MRI of Hepatocellular Carcinoma: Value of Washout in Transitional and Hepatobiliary Phases. Radiology. 2019;291(3):651-657.
13. Joo I, Lee JM, Lee DH, et al. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol. 2019;29(4):1724-1732.
14. Cha DI, Jang KM, Kim SH, et al. Liver Imaging Reporting and Data System on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur Radiol. 2017;27(10):4394-4405.
15. Hwang SH, Park S, Han K, et al. Optimal lexicon of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of hepatocellular carcinoma modified from LI-RADS. Abdom Radiol (NY). 2019;44(9):3078-3088.
Figures