0314
Free-Breathing Radial Imaging using a Pilot-Tone RF Transmitter for Detection of Respiratory Motion
Eddy Solomon1, Thomas Vahle2, Jan Paska1, Kai Tobias Block1, Daniel K. Sodickson1, Fernando Boada1, and Hersh Chandarana1
1Radiology, New York University School of Medicine, New York, NY, United States, 2Siemens Healthcare GmbH, Erlangen, Germany
1Radiology, New York University School of Medicine, New York, NY, United States, 2Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
The sensitivity of MRI sequences to motion impairs their reliability and diagnostic utility for examining the chest and abdomen. Established motion-compensation techniques are not accurate enough, come at the cost of patient comfort, and are limited by the MR imaging parameters. Here, we demonstrate a novel approach that detects respiratory signal from the amplitude modulation of a transmitted RF reference signal, termed ‘pilot-tone’ (PT). We show how the use of this simple RF transmitter, with its small dimensions, high sampling rate, and low interference with the MR acquisition, can produce motion corrected-images under free-breathing conditions.
INTRODUCTION
The slow acquisition speed of MRI can limit its applicability for examination of the chest and abdomen, often leading to motion artifacts caused by physiological movements such as cardiac and respiratory motion (1). External sensors (2) such as respiratory belts have traditionally been used for triggering the acquisition. However, this approach is oftentimes unreliable and tends to suffer from poor sensitivity. Self-navigation (3), on the other hand, extract motion information from k-space center, which introduces a dependency on imaging parameters (e.g. TR, number of slices) and can be too slow for certain types of physiological motion. In this work, we demonstrate an alternative approach which provides respiratory information with high temporal resolution. A reference RF signal (4), termed “pilot tone” (PT), is generated by a small radiofrequency transmitter and received by the MR system during each readout. Because the PT amplitude is modulated by the subject’s breathing pattern, a respiratory signal can be derived. The PT approach was tested using a radial MR sequence on human volunteers, and was compared to conventional methods for motion correction.METHODS
Experiment setup: All data were acquired on a 3T Prisma system (Siemens Healthcare, Erlangen, Germany) using a body coil array. The study protocol included a free-breathing radial stack-of-stars 3D GRE (RAVE) sequence with golden-angle acquisition. It was tested on 15 healthy human volunteers. RAVE imaging parameters included oversampling factor=2, TR/TE=4.0/1.7ms, BW=500Hz/pixel, 240 slices, 800 radial views, 1.6mm isotropic resolution.Principle: A prototype PT transmitter is a small device placed outside the MR bore with no direct contact with the patient. Physiological movement of the subject causes coil load variations, resulting in modulations of the PT signal detected by the MR receive coil. The PT signal frequency is controlled so that it is imprinted outside the object in image space. To avoid interference between the PT signal and the MR signal (center frequency at 3T = 123.25 MHz), the PT is tuned to transmit ~100 kHz off-resonance. The separation between the imaged object and the pilot-tone (Fig. 1a) is noticeable after FT along the frequency domain. The image (Fig. 1b) and PT (Fig. 1c) information can be separated and a respiratory signal can be extracted by a fit in the frequency domain:$$A\cdot\exp\left(-i\cdot2\pi\cdot\ f\cdot\ t\right)$$ where $$$ f$$$ is the PT frequency and $$$ A$$$ is the complex amplitude signal, which modulates in accordance with the respiration (Fig. 1d). A peak-detection algorithm calculates the PT’s frequency based on the strongest coil and then uses this frequency to calculate the coefficient ($$$A$$$) and its modulation over time for the rest of the coils.
Workflow: During experiments, the PT transmitter is placed outside the MR bore, resulting in a unique signal imprinted in the image data prior to processing (Fig. 2a). The breathing-related PT signal modulation, picked up by each receive coil, is extracted by the peak-detection algorithm and processed by principal component (PCA) analysis, resulting in one respiratory signal from all coils (Fig. 2b). As the next step, the respiratory signal is exploited by an eXtra-Dimensional (XD) reconstruction pipeline (3), which bins the continuously acquired radial data into different respiratory states from inhale to exhale (Fig. 2c).
RESULTS AND DISCUSSION
When comparing respiratory signals from the respiratory belt (Fig. 3a, blue), self-navigation (Fig. 3a, red), and pilot tone (Fig. 3a, green), similar patterns were seen for self-navigation and PT, but the belt signal differed from those patterns. The belt is an analog device that is sensitive to the patient setup, often leading to distorted/clipped signal as experienced here. Similarity between the PT and k-space center signals was noted whether the subject was breathing steadily (Fig. 3b) or unsteadily (Fig. 3c). To reduce dimensionality and noise, PCA analysis, second-order blind identification (SOBI) (5), and coil clustering (6) algorithms were used (Fig. 4a) to process the signals recorded by all MR coils. Respiratory signals based on k-space center (Fig. 4a, pink) missed some respiratory information while PT showed better signal recurrence when processed by the different methods. This result has a direct impact on how motion affects the final images (Fig. 4b), revealing clearer anatomical details like the pancreas and the abdominal wall when using the PT. Moreover, it also influences the image quality along the different respiratory motion states. In a 3D view of another volunteer, use of the PT signal resulted in less motion blurring especially at the liver tip and in the center of liver (Fig. 4c). To further illustrate the reliability of PT, a video of zero-angle acquisitions after Fourier transformation shows how the PT signal (blue) is synchronized with the actual breathing of the subject (Fig. 5).CONCLUSION
A novel device for tracking respiratory motion, called a pilot tone, showed fewer motion artifacts when compared to conventional k-space self-navigation and respiratory belts. The small dimensions (8 cm) and high sampling rate associated with the PT, offer great potential for tracking breathing signals. Additionally, since the pilot-tone retrieves its information separately from the MR data, it is well suited to tracking motion during the injection of contrast material, which is not possible with k-space-based signals due to spatially varying contrast uptake.Acknowledgements
We acknowledge support from NIH grant P41 EB0171813 and R01 5R01EB018308.References
- Havsteen I, Ohlhues A, Madsen KH, Nybing JD, Christensen H, Christensen A. Are Movement Artifacts in Magnetic Resonance Imaging a Real Problem?-A Narrative Review. Front Neurol 2017;8:232.
- Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. J Magn Reson Imaging 2015;42(4):887-901.
- Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016 Feb;75(2):775-88.
- Speier P, Fenchel M, Rehner R, PT-Nav: a novel respiratory navigation method for continuous acquisitions based on modulation of a pilot tone in the MR-receiverProc. ESMRMB 2015, 128: 97-98.
- Belouchrani A, AbedMeraim K, Cardoso JF, Moulines E. A blind source separation technique using second-order statistics. Ieee T Signal Proces 1997;45(2):434-444.
- Zhang T, Cheng JY, Chen YX, Nishimura DG, Pauly JM, Vasanawala SS. Robust Self-Navigated Body MRI Using Dense Coil Arrays. Magnetic Resonance in Medicine 2016;76(1):197-205.
Figures
Figure
1. k-space
information after FT of stacked radial views: (a) the pilot-tone and image data
which can be separated to (b) image or (c) pilot-tone data by a peak detection
algorithm. (d) In the presence of breathing, the pilot-tone modulation over
time can generate a breathing curve (in green).
Figure
2. Pilot-tone
processing steps: (a) The pilot-tone transmitter is attached
outside
the MR
bore,
imprinting its signal in image data. (b) The breathing-related PT
signal modulation, picked up by each receive coil, is extracted by a
peak-detection algorithm and processed by principal component (PCA) analysis.
(c) As a next step, the respiratory signal is exploited by the eXtra-Dimensional
(XD) image reconstruction pipeline, which bins the continuously acquired radial
data into different respiratory states from inspiration (“inhale”) to
expiration (“exhale”).
Figure 3. (a)
Comparing respiratory signals recorded by the bellow belt (blue), k-space
center (red) and pilot-tone (green). Next, comparing only the k-space center
(red) and pilot-tone (green) for (b) steady and (c) unsteady
breathing. Respiratory data
is overlaid on top of Fourier transform of k-space center lines.
Figure 4. (a)
Pilot-tone (PT) and k-space center (CK) signals processed by SOBI, PCA and
coil-clustering (coilC)
signal processing algorithms. (b) MR data sorted using PT signal showed an
advantage in image quality over that sorted using k-space center signal,
revealing clearer anatomical details in, e.g., the pancreas and the abdominal
wall. (c) In a 3D view of another volunteer, data sorted using PT signal showed
less motion blurring especially at the liver tip and in the center of the liver
for different respiratory states.
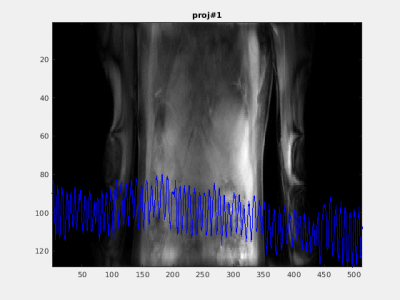
Figure
5. Pilot-tone was found to be in
good agreement with the physiological motion of the subject acquired in
zero-angle projections.