0313
Ungated, Motion Robust, Simultaneous Cardiac and Liver T2* Quantification via Rosette k-Space Sampling1Radiology, Stanford University, Palo Alto, CA, United States, 2Electrical Engineering, Stanford University, Palo Alto, CA, United States, 3Electrical Engineering, University of California Berkeley, Berkeley, CA, United States, 4Radiology, University of California San Francisco, San Francisco, CA, United States
Synopsis
In this work we introduce a novel method for T2* determination using rosette k-space sampling and locally low-rank reconstruction. This approach produced comparable T2* quantitation with higher spatial resolution, fewer motion artifacts and lessened variability without the use of gating. This approach offers a child-friendly, rapid, free-breathing, comprehensive assessment of liver and cardiac iron.
Introduction
Over the last 20 years, quantitative T2* MRI has replaced painful biopsy, transformed the clinical management and reduced cardiac-related mortality in iron overloaded patients. Despite the clear success, clinical MRI based iron assessment is largely unchanged and usually consists of both a volumetric, breath-held liver acquisition and a short-axis, single slice cardiac gated acquisition. Though effective in ideal conditions, patient motion, fast heart rates and gating failures corrupt T2* measurements1, leading to long scan time, repeated scans and sedation in children.Therefore, the goal of this work was to develop and validate a novel, ungated, free-breathing, non-Cartesian approach to multi-echo, T2* imaging using rosette k-space trajectories and a model-based reconstruction framework.
Theory
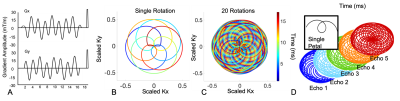
Rosette trajectories are self-crossing, flower-like trajectories following the equation: 1) k(t)=kmaxsin(w1t)exp(iw2t), where w1 is the rotational frequency and w2 is the center sampling frequency. When w2 is larger than w1; wide looping Class II trajectories are formed which have been shown to have better hardware utilization and incoherence properties for compressed sensing reconstructions3. By performing a multi-shot acquisition and separating k-space regions by successive center crossings, a multi-echo data set for T2* assessment is obtained (Figure 1).To compare the precision and accuracy of this novel rosette approach to the clinical standard, Cartesian multi-echo sequences, the following methods were performed.
Methods
Imaging was performed on a 1.5T, GE Signa 450W MRI system with a 32 channel cardiac coil. For the Cartesian sequence, a gated, unipolar, 8 echo, multi-echo GRE sequence was used with the initial echo time of 1.5ms and echo spacing of 2.0ms. Other sequence parameters included FA 25 degrees, TR 15.7 ms, 40 cm field of view, 1.5 mm in-plane resolution and 8 mm slice thickness. Total scan time ranged from 15-20 seconds depending on heart rate.A spoiled GRE, ungated, rosette pulse sequence with a 15 degree FA, a repetition time of 17 ms and w2/w1 ratio of 2.2 was used. The sequence was constrained to a maximum slew rate of 75 mT/m/s and gradient amplitude of 40 mT/m to reduce eddy current and concomitant gradient related artifacts. A single repetition was rotated by the golden angle, 137.5°, and repeated 800 times for a comfortable breath held scan of 15 seconds. Five echoes were reconstructed with a sampling window of 750 samples per segment. An empirical gradient delay of 2.4ms on the x and y physical gradient axis and 0.6ms on the z physical gradient axis was applied retrospectively. A locally-low rank reconstruction was performed using BART4. Magnitude based mono-exponential fitting was used to determine the T2* value for both the clinical standard and rosette multi-echo data.
Phantoms
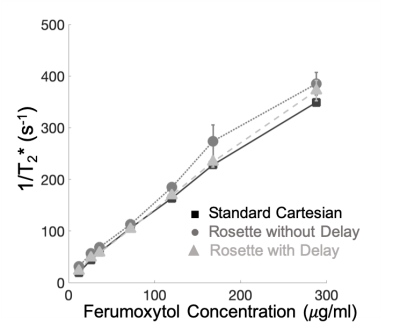
Six phantoms containing distilled water, 2% carrageenan by mass5 and variable concentrations of ferumoxytol6 (26, 36, 72, 120, 168, 288 mg/mL) were constructed in house. The T2* phantoms were submerged in a room temperature water bath to limit susceptibility artifacts. Cartesian and rosette T2* estimates were compared using Bland-Altman statistics.
In Vivo
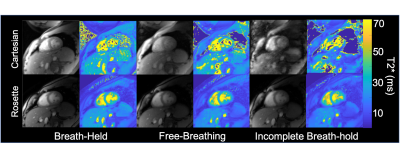
All human studies were IRB approved and informed consent and assent was obtained. In subjects, a single, mid short axis slice was acquired, with ECG gating for Cartesian exams. Rosette and cartesian T2* motion sensitivity testing was performed in 8 healthy volunteers. A diastolic self-gated and continuous time-averaged rosette reconstruction and T2* estimates were compared. Test retest reproducibility was performed by repeating 3, breath-held Cartesian and rosette scans pairs. A free breathing and failed breath-held exam was performed to assess motion sensitivity between Cartesian and rosette T2* estimates. During the failed breath-hold, volunteers were instructed to hold their breath for 5-10 seconds then resume, normal tidal breathing. Coefficient of variation analysis (s/m) was used to determine bias and limits of agreement in experimental data.
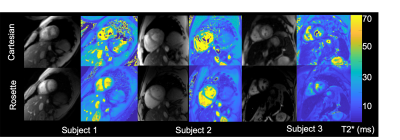
A total of 12 patients undergoing clinically indicted MRI iron assessment also had a single, breath-held Cartesian and rosette acquisition.
Results
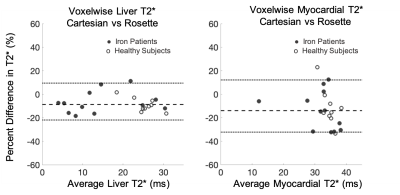
Phantoms The measured 1/T2* was linear with ferumoxytol iron concentration up to a measured concentration of 288 mg/mL (r2=0.999). Bland Altman Cartesian and rosette T2* estimates demonstrated an average bias of 2.9%±4.2% following gradient delay correction. In Vivo Diastolic self-gated and time-averaged rosette T2* estimates were not statistically different (p=0.24), with pair-wise differences of -1.1 ± 0.9ms (p=0.24) for the liver and 0.1 ± 0.8ms (p=0.90) for the myocardium. The cardiac test-retest coefficient of variation was 4.6%±1.1% and 2.6%±1.4% for Cartesian and rosette T2* respectively (p=.87). Figure 3 displays representative Cartesian and rosette images under different breathing conditions. Rosette images produced less T2* error during free-breathing and failed breath-held conditions. Figure 4 displays representative Cartesian and rosette images in patients with iron loading and limits of agreement in all subjects (Figure 5). The difference in Cartesian and rosette liver T2* was -1.4 ± 1.7 ms (p<0.001) and myocardial T2* was -4.5 ± 5.4 ms (p<0.001) (Figure 5).Discussion
In this work we introduce a novel method for T2* determination using rosette k-space sampling and locally low-rank reconstruction. This approach produced comparable T2* quantitation with higher spatial resolution, fewer motion artifacts and lessened variability without the use of gating. This approach offers a child-friendly, rapid, free-breathing, comprehensive assessment of liver and cardiac iron.Acknowledgements
The authors would like to acknowledge: NIH R01 EB009690 and NIH R01 EB026136, and GE precision healthcare for support.
References
1. Kellman P, Xue H, Spottiswoode BS, et al. Free-breathing T2* mapping using respiratory motion corrected averaging. J Cardiovasc Magn Reson. 2015;17(1):3.
2. Noll DC. Multishot rosette trajectories for spectrally selective MR imaging. IEEE Trans Med Imaging. 1997;16(4):372-377.
3. Li Y, Yang R, Zhang C, Zhang J, Jia S, Zhou Z. Analysis of generalized rosette trajectory for compressed sensing MRI. Med Phys. 2015;42(9):5530-5544.
4. Martin Uecker FO, Jonathan Tamir, Dara Bahri, Patrick Virtue, Joseph Cheng, Tao Zhang, Michael Lustig. Berkeley Advanced Reconstruction Toolbox. In. Proceedings International Society of Magnetic Resonance in Medicine2015.
5. Yoshimura K, Kato H, Kuroda M, et al. Development of a tissue-equivalent MRI phantom using carrageenan gel. Magn Reson Med. 2003;50(5):1011-1017.
6. Knobloch G, Colgan T, Wiens CN, et al. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Invest Radiol. 2018;53(5):257-263.
Figures